
Visual Abstract: Paracetamol and Ibuprofen and Morphine Use After Total Hip Replacement


EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, FEBRUARY 15, 2019
Media advisory: To contact corresponding study author Stefanos N. Kales, M.D., M.P.H., email Chris Sweeney at csweeney@hsph.harvard.edu. The full study is linked to this news release and a visual abstract is below.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2018.8341
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: A study of male Indiana firefighters suggests that push-up capacity may be associated with lower risk of cardiovascular disease (CVD) events, including coronary artery disease, heart failure or sudden cardiac death. In this observational study of more than 1,100 firefighters, incidence of CVD was reduced for each increase in 10 push-ups. However the association between push-up capacity and reduced CVD remained only for the 21-to-30 push-up category after accounting for age and body mass index (BMI) and wasn’t evident after accounting for maximal oxygen consumption, a physiologic measure of fitness. The findings suggest that low push-up capacity is a risk factor for CVD, but not independent of age, BMI, and oxygen consumption. Researchers caution the results may not generalize to others, including women and people who are inactive, because the study group consisted of middle-aged men who were active on the job. Larger studies with more diverse groups of people are needed to understand if push-up capacity can be used as an objective clinical tool to help assess patients and if it can provide useful information beyond standard assessments of age and BMI.
Authors: Stefanos N. Kales, M.D., M.P.H., Harvard T.H. Chan School of Public Health, Boston, Massachusetts, and coauthors
Visual Abstract:

(doi:10.1001/jamanetworkopen.2018.8341)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, FEBRUARY 13, 2019
Media advisory: To contact corresponding author Gabriella Gobbi, M.D., Ph.D., email Julie Robert at julie.robert@muhc.mcgill.ca. The full study is linked to this news release.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamapsychiatry/fullarticle/2723657?guestAccessKey=ca2d5287-277b-497a-a025-3826723adac1&utm_source=JAMA Network&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=21319
Bottom Line: Marijuana is commonly used by teenagers but not much is known about how that use might impact mood and risk of suicide later in life. This study analyzed the combined the results of 11 studies with about 23,300 people and found marijuana use during adolescence before age 18 was associated with increased risk of depression and suicidal thoughts or attempts during young adulthood between the ages of 18 and 32. There was no similar association with anxiety. The findings highlight the importance of efforts aimed at educating teenagers about the risks of using marijuana.
Authors: Gabriella Gobbi, M.D., Ph.D., McGill University, Montreal, Canada, and coauthors
(doi:10.1001/jamapsychiatry.2018.4500)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, FEBRUARY 13, 2019
Media advisory: To contact corresponding author Amanda H. Kerbrat, M.S.W., email Leila Gray at leilag@uw.edu. The full study, editorials and podcast are linked to this news release.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamapsychiatry/fullarticle/2723658?guestAccessKey=45f04731-0ab6-43ed-9da4-3f08f2885651&utm_source=JAMA Network&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=21319
Bottom Line: A randomized clinical trial of about 650 U.S. Army soldiers and Marines showed inconsistent results for a suicide prevention intervention that supplemented standard care with caring text messages to reduce suicidal thoughts and behaviors. Two accompanying editorials discuss the inexpensive intervention and potential reasons that could help to explain the uncertain results in a military population.
Authors: Amanda H. Kerbrat, M.S.W., University of Washington, Seattle, and coauthors
(doi:10.1001/ jamapsychiatry.2018.4530 )
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, FEBRUARY 13, 2019
Media advisory: To contact corresponding author Dorry L. Segev, M.D., Ph.D., email Raigan Wheeler at rwheel13@jhmi.edu. The full study and commentary are linked to this news release.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamapsychiatry/fullarticle/2723658?guestAccessKey=45f04731-0ab6-43ed-9da4-3f08f2885651&utm_source=JAMA Network&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=21319
Bottom Line: More than 10 percent of patients waiting for a liver transplant die each year. This observational study looked at trends in the transplantation of livers from older donors (70 and older) and outcomes in recipients of these older livers from 2003 to 2016. There was a decrease in the use of liver grafts from older donors despite improvements in liver graft loss and death among recipients of these older liver grafts. The study included 4,127 liver grafts from older donors and 3,350 liver-only recipients of these older liver grafts, and 78,990 liver grafts from younger donors (18 to 69) and 64,907 liver-only recipients of these younger liver grafts. A limitation of the study was the inability to determine whether the improvement in outcomes was associated with improved post-transplant care or improved older donor candidate selection. The findings suggest it may be reasonable to expand the donor pool with broader use of liver grafts from older donors.
Authors: Dorry L. Segev, M.D., Ph.D., Johns Hopkins University School of Medicine, Baltimore, and coauthors
(doi:10.1001/jamasurg.2018.5568)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, FEBRUARY 11, 2019
Media advisory: To contact corresponding author Daniel G. Whitney, Ph.D., email Kara Gavin at kegavin@med.umich.edu. The full study is linked to this news release.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time: https://jamanetwork.com/journals/jamapediatrics/fullarticle/2724377?guestAccessKey=f689aa19-31f1-481d-878a-6bf83844536a
Bottom Line: An estimated 7.7 million children in the United States (16.5 percent) have at least one mental health disorder and about half didn’t receive treatment from a mental health professional. National survey data were used to estimate how common mental health disorders were in children at the national and state levels, along with how common mental health care use was in children. An estimated 46.6 million children were included for analysis and prevalence estimates varied widely by state. For example, the prevalence of children with at least one mental health disorder ranged from 7.6 percent in Hawaii to 27.2 percent in Maine and the prevalence of children with a mental health disorder not treated or counseled by a mental health professional ranged from 29.5 percent in Washington, D.C., to 72.2 percent in North Carolina. Policy efforts to improve treatment across the states are needed.
Authors: Daniel G. Whitney, Ph.D., and Mark D. Peterson, Ph.D., of the University of Michigan, Ann Arbor
(doi:10.1001/jamapediatrics.2018.5399)
Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 5 P.M. (ET), MONDAY, FEBRUARY 11, 2019
Media advisory: To contact corresponding author Brandon A. Mahal, M.D., email Victoria Warren at victoria_warren@dfci.harvard.edu. The full study is linked to this news release.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time: https://jamanetwork.com/journals/jama/fullarticle/10.1001/jama.2018.19941
Bottom Line: National guidelines in 2010 began advocating conservative management of low-risk prostate cancer with active surveillance or watchful waiting (AS/WW) as an alternative to radiation to the treat the prostate or surgery to remove the entire prostate. This study examined trends in the management of localized prostate cancer among 165,000 men from 2010 to 2015 using data from a national database of cancer statistics. Use of AS/WW for men with low-risk localized prostate cancer increased from 14.5 percent in 2010 to 42 percent in 2015, becoming the most common management approach. Use of AS/WW increased among men with intermediate-risk disease and remained stable among those with high-risk disease. Surgery to remove the entire prostate declined among men with low-risk disease but increased among patients with higher-risk disease. A limitation of the study is the lack of data on AS/WW compliance.
Authors: Brandon A. Mahal, M.D., Dana-Farber Cancer Institute, Brigham and Women’s Hospital, Boston, and coauthors.
(doi:10.1001/jama.2018.19941)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, FEBRUARY 11, 2019
Media advisory: To contact corresponding author Sylvana M. Côté, Ph.D., email sylvana.cote.1@umontreal.ca. The full study is linked to this news release.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time: https://jamanetwork.com/journals/jamapediatrics/fullarticle/2724382?guestAccessKey=6c5b881d-e164-40ef-9784-7419ff54d8ba
Bottom Line: In a study of 920 boys from low-socioeconomic neighborhoods in Montreal, Canada, teacher ratings of inattention in kindergarten at ages 5 and 6 were associated with lower earnings as adults 30 years later, while increased ratings on prosocial behavior (such as helping, sharing and cooperating) were associated with higher earnings after accounting for child IQ and family adversity. Average personal earnings in adulthood were about $29,000 and an increase in inattention ratings as a child was associated with a decrease in earnings of about $1,300 and better ratings on prosocial behavior were associated with increased earnings of about $400. Teacher ratings on behaviors of hyperactivity, opposition and aggression weren’t associated with earnings in this observational study, which used earning data from government tax records. A limitation of the study is that it cannot explain causal reasons behind the observed associations.
Author: Sylvana M. Côté, Ph.D., of the Université de Montreal, Canada, and coauthors
(doi:10.1001/jamapediatrics.2018.5375)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, FEBRUARY 11, 2019
Media advisory: The full studies and commentary are linked to this news release.
Bottom Line: JAMA Internal Medicine is publishing four opioid-related articles (an original investigation, invited commentary and two research letters) that report on racial/ethnic and income disparities in the prescription of opioids and other other controlled medications in California, racial differences in opioid overdose deaths in New York, and county-level opioid prescribing in the United States.
Want to embed links to these articles in your story? These full-text links will be live at the embargo time:
Original Investigation: Assessment of Racial/Ethnic and Income Disparities in the Prescription of Opioids and Other Controlled Medications in California https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2723625?guestAccessKey=7fe163de-0ce6-4464-bf27-3c0dfafbc437
Invited Commentary: Opioid Prescribing Trends and the Physician’s Role in Responding to the Public Health Crisis https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2723622?guestAccessKey=eaeac693-8773-4ecc-8353-6327c9902121
Research Letter: County-Level Opioid Prescribing in the United States, 2015 and 2017 https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2723623?guestAccessKey=fbc1862d-97ca-4f52-9905-1edcf73387a2
Editor’s Note: Please see the articles for additional information, including other authors, author contributions and affiliations, financial and conflict of interest disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or emailmediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, FEBRUARY 12, 2019
Media advisory: To contact the U.S. Preventive Services Task Force, email the Media Coordinator at Newsroom@USPSTF.net or call 202-572-2044. The full report and related articles are linked to this news release.
Want to embed a link to this report in your story? This full-text link will be live at the embargo time and all links to all USPSTF articles remain free indefinitely: https://jamanetwork.com/journals/jama/fullarticle/10.1001/jama.2019.0007
Bottom Line: The U.S. Preventive Services Task Force (USPSTF) recommends clinicians provide counseling interventions to pregnant and postpartum women at increased risk of depression or refer patients to those services.
Background: The USPSTF routinely makes recommendations about the effectiveness of preventive care services. This latest statement is a new recommendation on interventions to prevent perinatal depression, which is the development of a depressive disorder during pregnancy or after childbirth. Depression is one of the most common complications during pregnancy and after childbirth, and it can have adverse effects on both women and children.
The USPSTF Concludes:

(doi:10.1001/jama.2019.0007)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
Note: More information about the U.S. Preventive Services Task Force, its process, and its recommendations can be found on the newsroom page of its website.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), THURSDAY, FEBRUARY 7, 2019
Media advisory: To contact corresponding author Yara Haridy, M.S., email yara.haridy@mfn-berlin.de. The full study is linked to this news release.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamaoncology/fullarticle/2723578?guestAccessKey=36a3caee-1474-4c66-88e0-e38dc4e8304d
Bottom Line: This research letter documents bone cancer in a 240-million-year-old stem-turtle from the Triassic period, helping to provide more data about the history of cancer in tetrapod evolution. This is a case study about a highly malignant bone tumor on the femur of a shell-less stem-turtle. The appearance of the tumor in the fossilized specimen conforms with present-day periosteal osteosarcoma in humans.
Authors: Yara Haridy, M.S., of the Museum für Naturkunde, Berlin, Germany, and coauthors
Featured Image: The image shows the bone cancer (osteosarcoma) on the femur of the fossil stem-turtle. The circled area shows the extent of the mass.
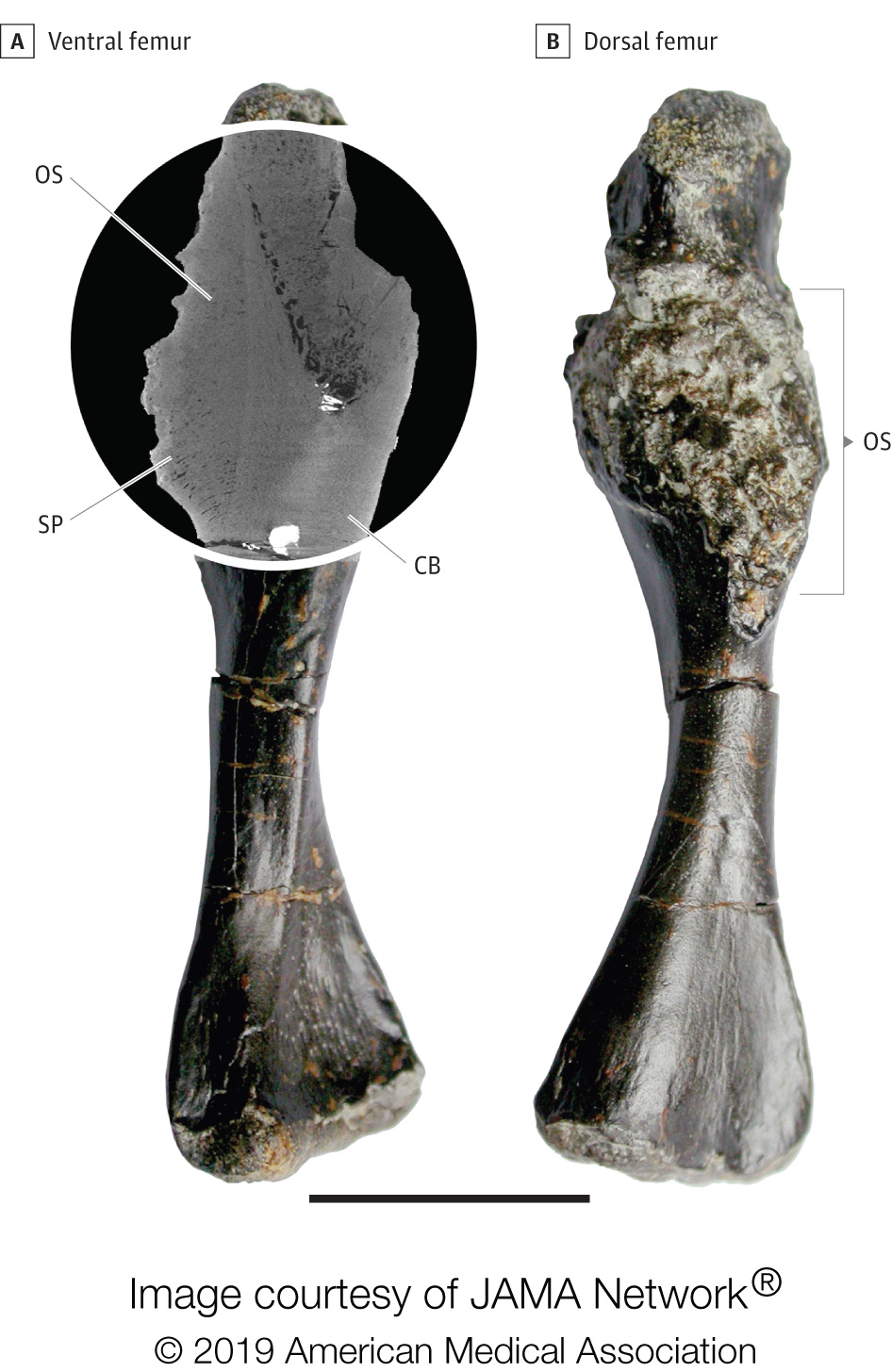
(doi:10.1001/jamaoncol.2018.6766)
Editor’s Note: The article includes conflict of interest disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), THURSDAY, FEBRUARY 7, 2019
Media advisory: To contact corresponding author Dong-Kyu Kim, M.D., Ph.D., email doctordk@naver.com. The full study and commentary are linked to this news release.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamaotolaryngology/fullarticle/2724230?guestAccessKey=9200fd5c-1371-46df-91ef-12048a2cf8a5
Bottom Line: Chronic rhinosinusitis is a common condition marked by sinus inflammation that can make breathing difficult and cause face pain or tenderness. The condition affects quality of life but whether it contributes to depression and anxiety in patients is unclear. This study of about 49,000 people in a South Korean insurance database examined the risk of depression and anxiety in chronic rhinosinusitis and depending on the type of chronic rhinosinusitis (with or without nasal polyps). Researchers report chronic rhinosinusitis was associated with an increased risk of depression and anxiety during 11 years of follow-up and that having nasal polyps was associated with a higher risk of depression and anxiety than chronic rhinosinusitis without nasal polyps. A limitation of the study is that it didn’t include information on smoking and alcohol use by participants and those factors could have influenced outcomes.
Authors: Dong-Kyu Kim, M.D., Ph.D., Hallym University College of Medicine, Chuncheon, Republic of Korea, and coauthors
(doi:10.1001/jamaoto.2018.4103)
Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, FEBRUARY 8, 2019
Media advisory: To contact corresponding study author Afton L. Hassett, Psy.D., email Kelly Malcom at kmalcom@med.umich.edu. The full study is linked to this news release and a visual abstract is below.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2723643?guestAccessKey=e0f7b001-bb32-4770-9b51-ebe3e787f9e6
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: Many veterans experience chronic pain after deployment. This study of almost 21,000 U.S. Army soldiers who deployed to Afghanistan or Iraq examined the association between feelings of optimism (such as expecting the best and believing good things will happen) before deployment and new reports of pain after deployment, including new back pain, joint pain and frequent headaches. Higher levels of optimism before deployment were linked with a lower likelihood of reporting new pain after deployment, even after accounting for demographic, military and combat factors. The findings suggest soldiers with low levels of optimism before deployment may benefit from programs designed to enhance feelings of optimism. There are limitations to interpreting the study results because researchers didn’t account for psychiatric disorders and assessments of pain were limited.
Authors: Afton L. Hassett, Psy.D., University of Michigan, Ann Arbor, Michigan, and coauthors
Visual Abstract: 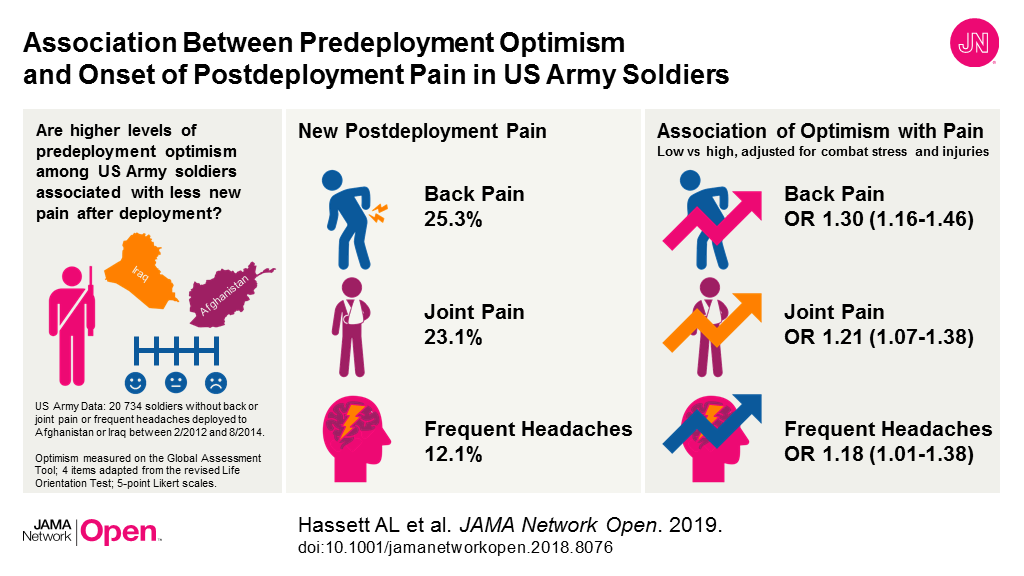
(doi:10.1001/jamanetworkopen.2018.8076)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, FEBRUARY 6, 2019
Media advisory: To contact corresponding author Erik Pettersson, Ph.D., email erik.pettersson@ki.se. The full study is linked to this news release.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamapsychiatry/fullarticle/2722846?guestAccessKey=6ca738e0-dc1a-4d8a-a853-7f93e29c27d5
Bottom Line: It is unclear if the associations between fetal growth as indicated by birth weight and later mental health conditions remain after taking into account family-related factors that could affect these conditions. This study included more than 500,000 pairs of siblings who were part of a register in Sweden since birth and were followed up to an average age of 27 years. After controlling for family-related factors that could influence the outcomes, lower birth weight (adjusted for gestational age) was associated with a small but significant increased risk for several psychiatric disorders, including attention-deficit/hyperactivity disorder, autism, obsessive-compulsive disorder and depression. A potential limitation of the study is that the registers only included individuals with more severe forms of these conditions.
Authors: Erik Pettersson, Ph.D., Karolinska lnstitutet, Stockholm, Sweden, and coauthors
(doi:10.1001/jamapsychiatry.2018.4342)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, FEBRUARY 6, 2019
Media advisory: To contact corresponding author James P. Byrne, Ph.D., M.D., email Laura Bristow at Laura.Bristow@sunnybrook.ca. The full study and commentary are linked to this news release.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamasurgery/fullarticle/2723267?guestAccessKey=b77f7f26-9bd6-4815-8540-d9448184de88
Bottom Line: Motor vehicle crashes are a leading cause of death and injury in the United States. Emergency medical service (EMS) response time is a factor with the potential to influence survival. This study examined EMS response times to motor vehicle crashes in nearly 2,300 U.S. counties from 2013 to 2015. Longer EMS response times in counties were associated with higher rates of motor vehicle crash mortality, after accounting for other important regional differences in EMS time intervals, access to trauma resources, traffic safety laws and how rural a county is. A significant proportion of fatalities (almost 10 percent in rural/wilderness areas and 14 percent in urban/suburban areas) were associated with prolonged county response times as defined by the median time (10 minutes or greater in rural/wilderness areas and 7 minutes or greater in urban/suburban areas). The authors interpret their findings to suggest that regional differences in EMS response time capabilities should be evaluated in efforts to improve trauma systems to reduce motor vehicle crash deaths. An important limitation of the study was the inability to capture regional differences in crash characteristics, which could have influenced the outcomes.
Authors: James P. Byrne, Ph.D., M.D., Sunnybrook Health Sciences Center, Toronto, and coauthors
(doi:10.1001/jamasurg.2018.5097)
Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, FEBRUARY 5, 2019
Media advisory: To contact corresponding author Niels Skipper, Ph.D., email nskipper@econ.au.dk. The full study is linked to this news release.
Want to embed a link to this study in your story? This link will be live at the embargo time: https://jamanetwork.com/journals/jama/fullarticle/10.1001/jama.2018.21819
Bottom Line: This observational study compared standardized test scores in reading and math for more than 630,000 Danish public school children with and without type 1 diabetes. Researchers found no significant difference in reading and math scores between the groups of children who were attending second, third, fourth, sixth and eighth grades in Denmark. The findings may not apply to other countries.
Authors: Niels Skipper, Ph.D., Aarhus University, Aarhus, Denmark, and coauthors.
(doi:10.1001/jama.2018.21819)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, FEBRUARY 4, 2019
Media advisory: To contact study author Lydia E. Pace, M.D., M.P.H., email Johanna Younghans at jyounghans@bwh.harvard.edu. The full study and commentary are linked to this news release.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2723071?guestAccessKey=0784ba9e-1dd9-46a3-92d7-9849b434a0e2
Bottom Line: Insertions of long-acting reversible contraceptive (LARC) methods increased in the 30 business days after the 2016 presidential election based on an analysis of data for a large group of commercially insured women. Industry and media reports after the 2016 election of Donald Trump described an increase in the utilization of LARC methods; one proposed reason was that women were concerned about access to contraceptives if the Patient Protection and Affordable Care Act (ACA) was repealed under the Trump administration. LARC methods (intrauterine devices and implants) can be effective for years at preventing pregnancy. This study of commercially insured women (more than 3.4 million in 2015 and more than 3.2 million in 2016) compared LARC utilization during the 30 days after the 2016 election with 30 days before the election and the same time period in 2015. In 2015, the average adjusted daily LARC insertion rate during the 30 business days before and including November 8 was 12.9 per 100,000 women compared with 13.7 per 100,000 women during the subsequent 30 days; the comparable averages before and after the 2016 election were 13.4 vs. 16.3 per 100,000 women. The authors acknowledge important limitations of their study including that they only studied women with commercial insurance.
Authors: Lydia E. Pace, M.D., M.P.H., of Brigham and Women’s Hospital, Boston, Massachusetts, and coauthors
Editor’s Note: The article contains conflict of interest disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or emailmediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, FEBRUARY 4, 2019
Media Advisory: To contact corresponding author Reisa A. Sperling, M.D., email Terri Janos at tjanos@partners.org. The full study is linked to this news release.
To place an electronic embedded link in your story: This link will be live at the embargo time: https://jamanetwork.com/journals/jamaneurology/fullarticle/10.1001/jamaneurol.2018.4693
Bottom Line: Growing evidence suggests women may be at increased risk of certain physiological changes associated with Alzheimer disease (AD). This study examined nearly 300 clinically normal adults (average age 74) for deposits in the brain of the protein tau, a marker of AD, as measured by positron emission tomography. Women showed more tau in a region of the brain than men, which was associated with individuals with greater amounts of plaque deposits of the β-amyloid peptide (Aβ), another marker of AD. These findings support other studies in identifying potential reasons for differences in risk for AD between men and women. The study population may limit the generalizability of these results.
Authors: Reisa A. Sperling, M.D., Massachusetts General Hospital, Harvard Medical School, Boston, and coauthors
(doi:10.1001/jamaneurol.2018.4693)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, JANUARY 30, 2019
Media advisory: To contact corresponding author Benjamin D. Levine, M.D., email Lori Soderbergh at Lori.Soderbergh@UTSouthwestern.edu. The full study, commentary and author interview are linked to this news release.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamacardiology/fullarticle/2722746?guestAccessKey=3c09c9c9-2972-4158-a259-62acd9f7c23c
Bottom Line: Some studies have suggested that people with high levels of physical activity way beyond current physical activity guidelines, such as marathon runners, can have significant build-up of calcium in the arteries of their heart called coronary artery calcification (CAC). But data are limited about the risk of death in these highly active people with CAC. This study included nearly 22,000 men (average age almost 52) with varying levels of self-reported physical activity and who underwent CAC scanning. Elevated levels of CAC were more common among highly active men but after a decade of follow-up they didn’t have an increased risk of death compared with less-active men. Men with the highest levels of physical activity, regardless of CAC level, had a lower rate of death than those with the lowest activity levels. This study was observational and doesn’t allow for causal interpretations of the findings.
Authors: Benjamin D. Levine, M.D., University of Texas Southwestern Medical Center, Dallas, and coauthors.
(doi:10.1001/jamacardio.2018.4628)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, JANUARY 29, 2019
Media advisory: To contact the U.S. Preventive Services Task Force, email the Media Coordinator at Newsroom@USPSTF.net or call 202-572-2044. The full report and related articles are linked to this news release.
Want to embed a link to this report in your story? This full-text link will be live at the embargo time and all links to all USPSTF articles remain free indefinitely: https://jamanetwork.com/journals/jama/fullarticle/2722778?guestAccessKey=a2298e7c-7d40-4328-b37a-fcd57f3af914
Bottom Line: The U.S. Preventive Services Task Force (USPSTF) reaffirms its recommendation for the use of an antibiotic ointment to prevent gonococcal eye infections in all newborns, a gonorrhea infection that is transmitted from the mother to the newborn during delivery.
Background: The USPSTF routinely makes recommendations about the effectiveness of preventive care services. This latest statement is a reaffirmation of its 2011 recommendation on prevention of gonococcal ophthalmia neonatorum, a gonorrhea infection of the eye in newborns. This infection can spread to the cornea and cause blindness as early as 24 hours after birth. In the absence of prevention, transmission rates of gonococcal infection from mother to newborn are 30 percent to 50 percent.
The USPSTF Concludes:

(doi:10.1001/jama.2018.21367)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
Note: More information about the U.S. Preventive Services Task Force, its process, and its recommendations can be found on the newsroom page of its website.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JANUARY 28, 2019
Media advisory: To contact study author David M. Levine, M.D., M.P.H., M.A,. email Johanna Younghans at jyounghans@bwh.harvard.edu. The full study and commentary are linked to this news release.
Want to embed a full-text link to this study in your story? This full-text link will be live at the embargo time: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2721037?guestAccessKey=0983f57a-7a4e-480a-b0b4-7bbcc2c6649b
(doi:10.1001/jamainternmed.2018.6716)
Editor’s Note: The article contains funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or emailmediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, JANUARY 29, 2019
Media advisory: To contact corresponding author Stephen W. Patrick, M.D., M.P.H., M.S., email Craig Boerner at craig.boerner@vumc.org. The full study and editorial are linked to this news release.
Want to embed a full-text link to this study in your story? This full-text link will be live at the embargo time: https://jamanetwork.com/journals/jama/fullarticle/2722771?guestAccessKey=2ea89e39-3977-4a39-9b38-9b96aef3d4d1
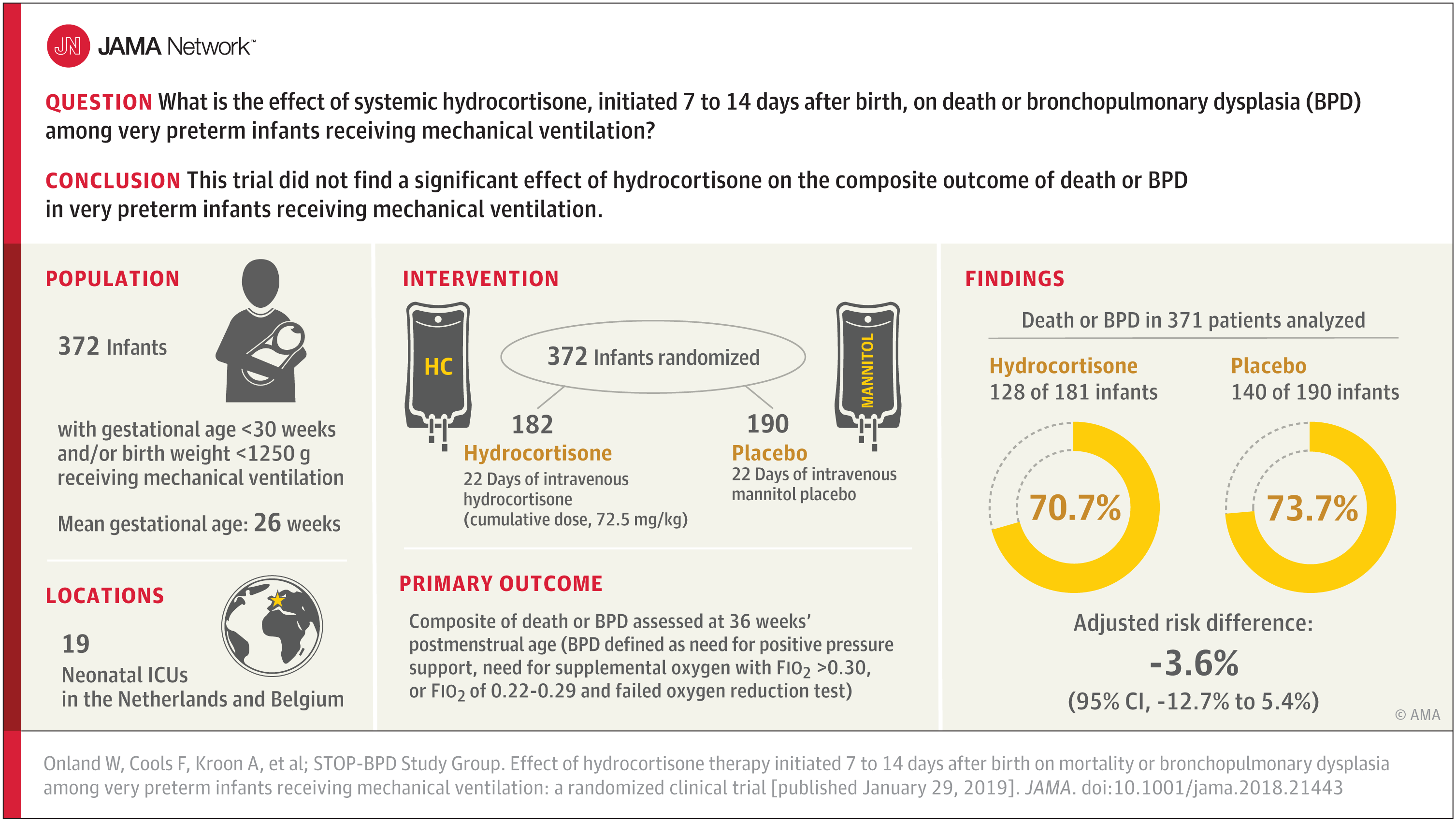
Bottom Line: Neonatal abstinence syndrome (NAS), which are symptoms that primarily occur in newborns exposed to opioids during pregnancy, has increased over the last two decades but there is limited information on its association with economic conditions or clinician supply. This study, which included 580 U.S. counties in eight states and 6.3 million births from 2009 to 2015, found higher rates of NAS at the county level to be associated with high rates of long-term unemployment and areas with a shortage of mental health clinicians. Neonatal abstinence syndrome rates were often highest in rural, remote counties. The design of the study does not allow for cause-and-effect interpretations of the findings.
Authors: Stephen W. Patrick, M.D., M.P.H., M.S., Vanderbilt University, Nashville, and coauthors.
(doi:10.1001/jama.2018.20851)
Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JANUARY 28, 2019
Media advisory: To contact corresponding author Jeff D. Williamson, M.D., M.H.S., email Marguerite Beck at marbeck@wakehealth.edu. The full study and editorial are linked to this news release and the visual abstract is below.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time: https://jamanetwork.com/journals/jama/fullarticle/2723256?guestAccessKey=f288411d-ef32-4812-b5a6-7da27b17151f
Bottom Line: Alzheimer disease and related dementias are projected to affect 115 million people worldwide by 2050. There are currently no proven treatments to reduce the risk of dementia and mild cognitive impairment (MCI). High blood pressure (hypertension) has been identified as a potentially modifiable risk factor for dementia and MCI in observational studies. In this randomized clinical trial that included about 9,400 adults age 50 or older with hypertension, participants were treated to achieve a systolic blood pressure goal of either less than 120 mm Hg (intensive treatment) or less than 140 mm Hg (standard treatment). The researchers found that intensive blood pressure control did not result in a significant reduction in the risk of probable dementia compared to those who received standard treatment. The study may have been underpowered for this outcome because of early termination of the study and fewer than expected cases of dementia.
Authors: Jeff D. Williamson, M.D., M.H.S., Wake Forest School of Medicine, Winston-Salem, North Carolina, and coauthors
Visual Abstract

(doi:10.1001/jama.2018.21442)
Editor’s Note: Please see the article for additional information, including author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JANUARY 28, 2019
Media advisory: To contact corresponding author Sheri Madigan, Ph.D., email Heath McCoy at hjmccoy@ucalgary.ca. The full study is linked to this news release.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time: https://jamanetwork.com/journals/jamapediatrics/fullarticle/2722666?guestAccessKey=879c6c87-141e-48f8-8c95-4d684600a644
Bottom Line: Many children spend more time on screens than is recommended. This study looked at whether more screen time was associated with lower scores in a measure of developmental milestones in children and it also looked at the opposite association of whether children with delays in development received more screen time to control challenging behavior. The study included about 2,400 typically developing children in Canada and found higher levels of screen time at ages 2 and 3 were associated with poorer performance on the developmental screening measure at ages 3 and 5. The opposite association wasn’t observed. A limitation of this observational study is that screen time behaviors in children may have changed since final data were collected in 2016. The authors recommend managing children’s screen time.
(doi:10.1001/jamapediatrics.2018.5056)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, JANUARY 25, 2019
Media advisory: To contact corresponding study author Tarak K. Trivedi, M.D., M.S., Enrique Rivero at erivero@mednet.ucla.edu. The full study and commentary are linked to this news release.
Want to embed a link to this study in your story?: This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2722574?guestAccessKey=c8d43986-1131-4af7-b3bc-a9f9415cd3b3
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: Nearly 250 patients ended up at two Southern California emergency departments with injuries associated with standing electric scooter use and few riders were wearing helmets. This observational study used medical record review to examine injuries associated with standing electric scooter use over a one-year period; 228 patients were injured as riders and 21 as nonriders. Nearly 11 percent of riders were younger than 18 and only about 4 percent of riders were documented as wearing helmets. Fractures, head injuries and soft-tissue injuries were the most common. Nearly all patients were discharged from the emergency department but 15 were admitted, including two with severe heard injuries. The authors suggest their findings may help to inform public policy around standing electric scooter use, a growing and cheap mode of transportation.
authors: Tarak K. Trivedi, M.D., M.S., University of California, Los Angeles, Los Angeles, California, and coauthors
(doi:10.1001/jamanetworkopen.2018.7381)
Editor’s Note: The article contains funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, JANUARY 25, 2019
Media advisory: To contact corresponding study author Tracie O. Afifi, Ph.D., email Chris Rutkowski at Chris.Rutkowski@umanitoba.ca. The full study is linked to this news release.
Want to embed a link to this study in your story? This full-text links will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2722572?guestAccessKey=3d62bf1f-9952-431a-b089-e74fbcf6929c
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: Harsh physical punishment (pushing, grabbing, shoving, slapping and hitting), maltreatment (physical abuse, sexual abuse, emotional abuse, emotional neglect, physical neglect and exposure to intimate partner violence) and a combination of the two during childhood were all associated with antisocial behaviors in adulthood among men and women. This observational study used data on about 36,000 adults in the general U.S. population. Authors suggest prevention efforts to eliminate harsh physical punishment and maltreatment in childhood should be a public health priority in an effort to reduce antisocial behavior among adults.
Authors: Tracie O. Afifi, Ph.D., University of Manitoba, Winnipeg, Canada, and coauthors
(doi:10.1001/jamanetworkopen.2018.7374)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, JANUARY 25, 2019
Media advisory: To contact corresponding author Sumit D. Agarwal, M.D., email Johanna Younghans at jyounghans@bwh.harvard.edu. The full study is linked to this news release and a visual abstract is below.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2018.7399
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: Benzodiazepines (tranquilizers) are a large class of drugs with lots of potential uses from treating anxiety to other conditions including insomnia, seizures and neuropathic pain. This study used nationally representative data to examine patterns in outpatient prescribing of benzodiazepines and included more than 386,000 ambulatory care visits from 2003 through 2015. The rate of ambulatory care visits where benzodiazepines were recorded nearly doubled over the time period from 3.8 percent to 7.4 percent. Primary care physicians accounted for about half of all visits with benzodiazepines. Authors suggest addressing prescribing patterns could help curb growing use of benzodiazepines amid increased benzodiazepine-related overdose deaths.
Authors: Sumit D. Agarwal, M.D., Brigham and Women’s Hospital, Boston, and Bruce E. Landon, M.D., M.B.A., M.Sc., Harvard Medical School, Boston.
Visual Abstract:

(doi:10.1001/jamanetworkopen.2018.7399)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or emailmediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, JANUARY 25, 2019
Media advisory: To contact corresponding author Noriaki Kurita, M.D., Ph.D., email kuritanoriaki@gmail.com. The full study is linked to this news release.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2018.7455
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: An earthquake and subsequent tsunami led to the Fukushima Daiichi Nuclear Power Plant disaster in Japan in 2011. This observational study examined associations between the earthquake and power plant disaster with birth rates in Fukushima City, the capital of the prefecture. There was an estimated 10 percent reduction in monthly birth rates in the first two years after the disaster but after that the trend in birth rates was similar to before the disaster, a finding the authors suggest may be indicative of rebuilding efforts. The study acknowledges the potential for underestimation of birth rates several years after the disaster.
Author: Noriaki Kurita, M.D., Ph.D., Fukushima Medical University Hospital, Fukushima City, Japan
(doi:10.1001/jamanetworkopen.2018.7455)
Editor’s Note: Please see the article for additional information, including author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or emailmediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, JANUARY 23, 2019
Media advisory: To contact corresponding author Kangmin Zhu, M.D., Ph.D., email Sarah Marshall at sarah.marshall@usuhs.edu. The full study is linked to this news release.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamasurgery/fullarticle/10.1001/jamasurg.2018.5113
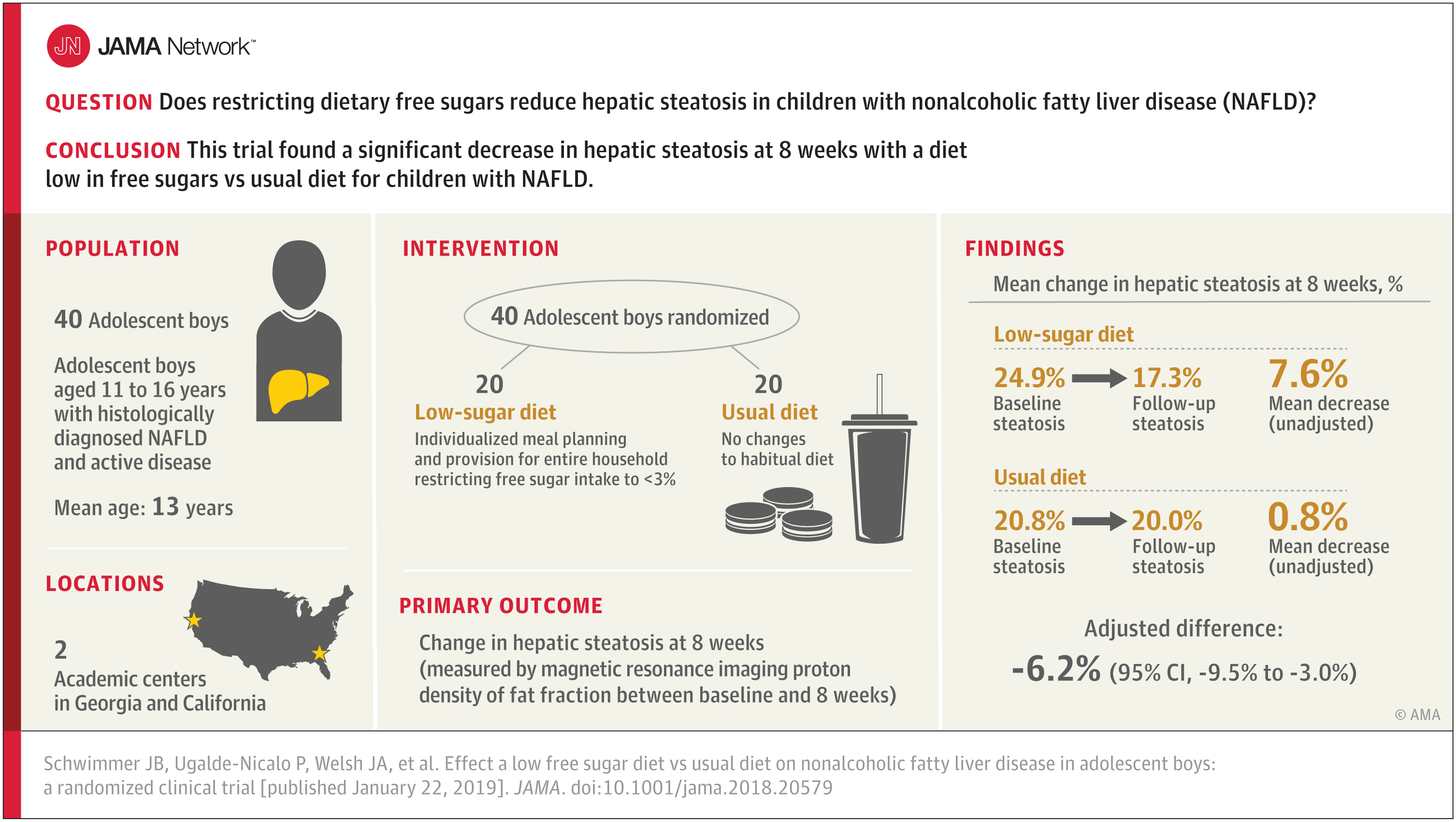
Bottom Line: Less access to care and lower insurance coverage are among the reasons for racial disparities in breast cancer survival in the United States. Eligible beneficiaries in the U.S. Military Health System have insurance and access to care. This study examined whether racial differences existed in time to surgery and whether any differences in that time might explain racial disparities in overall survival between nearly 1,000 black and 3,900 white women diagnosed with breast cancer in the Military Health System. Researchers report black women had greater estimated time to surgery than white women but that those delays don’t appear to explain racial disparities in overall survival. The clinical significance of differences in time to surgery in this study is unclear and more research is needed to understand racial disparities in breast cancer treatment and survival.
Authors: Kangmin Zhu, M.D., Ph.D., Uniformed Services University of the Health Sciences, Rockville, Maryland, and coauthors
(doi:10.1001/jamasurg.2018.5113)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, JANUARY 23, 2019
Media advisory: To contact corresponding author Aaron Reuben, M.E.M., email Karl Bates at karl.bates@duke.edu. The full study is linked to this news release.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamapsychiatry/fullarticle/10.1001/jamapsychiatry.2018.4192
Bottom Line: Millions of adults now entering middle age were exposed to high levels of lead as children, with childhood lead exposure linked to lower IQ, greater rates of child behavior problems, hyperactivity and antisocial behavior. This study included nearly 600 children in New Zealand who had their blood lead levels measured at age 11 and their mental health assessed periodically through age 38. Researchers found higher childhood blood lead levels were associated with more mental health problems throughout life and difficult adult personality traits such as being more neurotic, less agreeable and less conscientious. This was an observational study and it doesn’t allow for a cause-and-effect interpretation of the association between lead and the tested outcomes.
Authors: Aaron Reuben, M.E.M., Duke University, Durham, North Carolina, and coauthors
(doi:10.1001/ jamapsychiatry.2018.4192)
Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamapediatrics/fullarticle/2721243?guestAccessKey=9aa042b5-b0b9-4512-9924-a8e78140501e
Want to embed a link to this study in your story? This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamapediatrics/fullarticle/2721244?guestAccessKey=bdb9b43c-20d9-4c06-8195-789469c50510
Want to embed a link to this study in your story? This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamapediatrics/fullarticle/2721248?guestAccessKey=7998323e-6f5b-4d86-9c48-aa294f431c6f
Want to embed a link to this study in your story? This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamapediatrics/fullarticle/2721242?guestAccessKey=b3b36baf-7ff3-4450-b507-6d426ebdc4bb
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, JANUARY 22, 2019
Media advisory: To contact study author Norah A. Terrault, M.D., email Scott Maier at scott.maier@ucsf.edu. The full study and invited commentary are linked to this news release.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2720757?guestAccessKey=619ceb32-99d0-4da3-9d5f-2aebb18e56a3
Bottom Line: The proportion of liver transplants in the United States for alcohol-associated liver disease increased between 2002 and 2016, with much of the increase associated with a decrease in liver transplant for hepatitis C virus infection because of antiviral therapy. This observational study used data from the United Network for Organ Sharing for all liver transplants during the 15-year period and the national study group consisted of nearly 33,000 patients, including 9,438 patients with a diagnosis of alcohol-associated liver disease. Study findings suggest five-year survival after transplant was lower in patients with alcohol-associated liver disease. Authors suggest the increase in liver transplants for alcohol-associated liver disease may be related to changing attitudes about the length of sobriety needed for a transplant. The study relied on registry data so any conclusions are by association and not causal. Regional differences suggest dissimilar policies for liver transplant for alcohol-associated liver disease.
Authors: Norah A. Terrault, M.D., of the University of California, San Francisco, and coauthors
(doi:10.1001/jamainternmed.2018.6536)
Editor’s Note: The article contains funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or emailmediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, JANUARY 22, 2019
Media advisory: To contact corresponding author Sean L. Zheng, B.M., B.Ch., M.A., M.R.C.P., email sean.zheng@nhs.net. The full study and editorial are linked to this news release.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time https://jamanetwork.com/journals/jama/fullarticle/2721178?guestAccessKey=b6c6e6e2-80a8-449e-9278-a863ceaa9c67
Bottom Line: This study analyzed combined results from 13 randomized clinical trials with more than 164,000 participants to assess aspirin use with the prevention of cardiovascular events and bleeding in people without cardiovascular disease. Results suggest aspirin use was associated with lower risk (absolute risk reduction of 0.38 percent) for cardiovascular events (a composite of cardiovascular death, nonfatal heart attack and nonfatal stroke) and increased risk of major bleeding (absolute risk increase of 0.47 percent). The role of aspirin for the primary prevention of cardiovascular events has remained controversial because of an increased risk for bleeding. This study (a meta-analysis that combines the results of multiple studies identified in a systematic review) is limited by the availability and quality of reported data.
Authors: Sean L. Zheng, B.M., B.Ch., M.A., M.R.C.P., Imperial College London, and Alistair J. Roddick, B.Sc., King’s College London, United Kingdom
(doi:10.1001/jama.2018.20578)
Editor’s Note: Please see the article for additional information, including author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, JANUARY 22, 2019
Media advisory: To contact corresponding author Marsha Campbell-Yeo, Ph.D., email Terry Murray-Arnold at tmurraya@dal.ca. The full study and editorial are linked to this news release.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamapediatrics/fullarticle/2721241?guestAccessKey=34c29b3e-c3a2-4648-8f01-47c06c2b67ab
Bottom Line: Neonatal abstinence syndrome describes symptoms (including jitteriness, high-pitched crying, sweating and diarrhea) that primarily occur in newborns exposed to opioids during pregnancy. Finding an optimal drug therapy to treat newborns for neonatal abstinence syndrome may reduce the length of treatment and hospital stay. This study analyzed combined results from 18 randomized clinical trials that included morphine, the standard of care in most hospitals, and other medications to treat newborns for neonatal abstinence syndrome. Buprenorphine was associated with the shortest length of treatment but there were considerable limitations in the findings and a large trial is required for wide-scale adoption.
Author: Marsha Campbell-Yeo, Ph.D., Dalhousie University School of Nursing, Halifax, Nova Scotia, Canada, and coauthors
(doi:10.1001/jamapediatrics.2018.5044)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, JANUARY 18, 2019
Media advisory: To contact corresponding study author Madeline Penn, B.S., B.A., email Michelle Spivak at Michelle.SpivakMelinger@va.gov. The full study, invited commentary and a summary podcast are linked to this news release.
Want to embed a link to this study in your story?: This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2720917?guestAccessKey=05b5223a-1756-4852-bd4f-f66f53d44e77
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: This study compared new appointment wait times in the U.S. Department of Veterans Affairs (VA) health care system with wait times in the private sector. Wait time data were for primary care, dermatology, cardiology or orthopedics at VA medical centers in 15 major metropolitan areas and private sector comparison data came from a published survey.
Authors: Madeline Penn, B.S., of the U.S. Department of Veterans Affairs, Washington, D.C., and coauthors
(doi:10.1001/jamanetworkopen.2018.7096)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, JANUARY 16, 2019
Media advisory: To contact corresponding author Karen E. Joynt Maddox, M.D., M.P.H., email Diane Williams at williamsdia@wustl.edu. The full study is linked to this news release.
Want to embed a link to this study in your story? This full-text link will be live at the embargo time https://jamanetwork.com/journals/jamacardiology/fullarticle/2720425?guestAccessKey=b464ef40-d3a5-48c5-85d1-3f2e0c2f8060
Bottom Line: Lack of insurance is associated with worse care and outcomes among adults hospitalized for a heart attack. It is unclear whether states that expanded Medicaid eligibility under the Patient Protection and Affordable Care Act in 2014 had an associated improvement in quality of care and outcomes among low-income patients hospitalized with a heart attack. This observational study included 325,000 patients younger than 65 who had been hospitalized for a heart attack and found that state Medicaid expansion was associated with a significant reduction in rates of uninsurance among these patients. Quality of care and outcomes, such as risk of death and a prolonged hospital stay, didn’t improve among low-income adults in expansion states compared with nonexpansion states. The registry used in this study enrolls patients who may not necessarily be representative of all hospitals in the U.S.
Authors: Karen E. Joynt Maddox, M.D., M.P.H., Washington University School of Medicine in St. Louis, and coauthors.
(doi:10.1001/jamacardio.2018.4577)
Editor’s Note: The article includes conflict of interest disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, JANUARY 18, 2019
Media advisory: To contact corresponding study author Scott E. Hadland, M.D., M.P.H., M.S., email Jenny Eriksen at jenny.eriksen@bmc.org. The full study, invited commentary and a summary podcast are linked to this news release and a visual abstract is below.
Want to embed a link to this study in your story?: This full-text links will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2720914?guestAccessKey=630f38c9-ac45-406f-8764-b04eef425ce7
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: This study examined the association between pharmaceutical company marketing of opioids to physicians and subsequent death from prescription opioid overdoses across U.S. counties. The study, which analyzed industry marketing information data and national data on opioid prescribing and overdose deaths, reports almost $40 million in opioid marketing was targeted to more than 67,500 physicians across more than 2,200 counties from August 2013 to December 2015. Increases in opioid marketing to physicians were associated with higher prescribing rates and subsequently more death from prescription opioid overdoses a year later in this analysis. This observational study can show only associations, not causation. Findings suggest opioid marketing to physicians may counter national efforts to reduce the number of opioids prescribed and policymakers might consider limits on those activities.
Authors: Scott E. Hadland, M.D., M.P.H., M.S., Boston Medical Center, Boston, Massachusetts, and coauthors
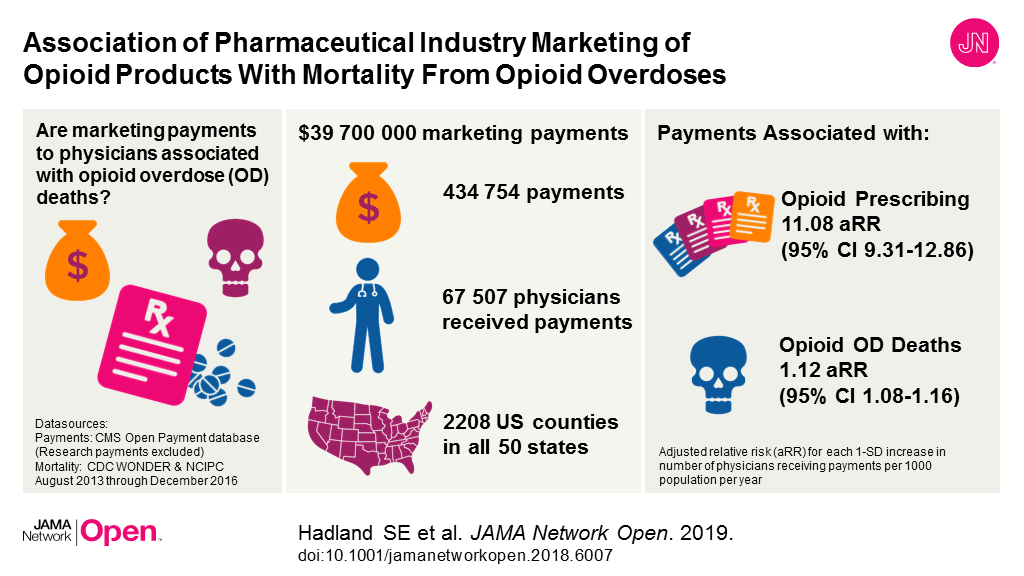
(doi:10.1001/jamanetworkopen.2018.6007)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, JANUARY 16, 2019
Media advisory: To contact corresponding author John S. Barbieri, M.D., M.B.A., email John Infanti at John.Infanti@pennmedicine.upenn.edu. The full study, an editorial and a podcast are linked to this news release.
Want to embed a link to this study in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jamadermatology/fullarticle/2720317?guestAccessKey=c0fe3bc7-c5b7-483f-9d05-1b97041a4aa3
Bottom Line: This study looked at trends over time in oral antibiotic prescribing by dermatologists using commercial insurance claims data for almost 986,000 courses of oral antibiotics prescribed by nearly 12,000 dermatologists. Overall, between 2008 and 2016, there was a decrease in antibiotic prescribing (from 3.36 to 2.13 courses per 100 visits with a dermatologist) and much of that decline came from a decrease in extended courses of antibiotics prescribed for acne and rosacea. However, prescribing of postoperative antibiotics after surgical visits increased (from 3.92 to 6.65 courses per 100 visits) and researchers suggest that practice be evaluated. The possibility of misclassification of diagnoses related to antibiotic prescriptions exists in this observational study.
Authors: John S. Barbieri, M.D., M.B.A., of the University of Pennsylvania Perelman School of Medicine, Philadelphia, and coauthors
(doi:10.1001/jamadermatol.2018.4944)
Editor’s Note: The article contains funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Listen to an interview with Erica S. Shenoy, M.D., Ph.D., of Massachusetts General Hospital, Boston, co-author of the JAMA study, “Evaluation and Management of Penicillin Allergy.” The podcast is available for listening and download on this page.
A summary video and visual abstract are available on this page for the study, “Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis,” by Samuel P. Costello, M.B.B.S., Queen Elizabeth Hospital, Woodville, Australia, and coauthors. The video can be embedded on your website by copying and pasting the HTML code below. To download the video, email mediarelations@jamanetwork.org for information.
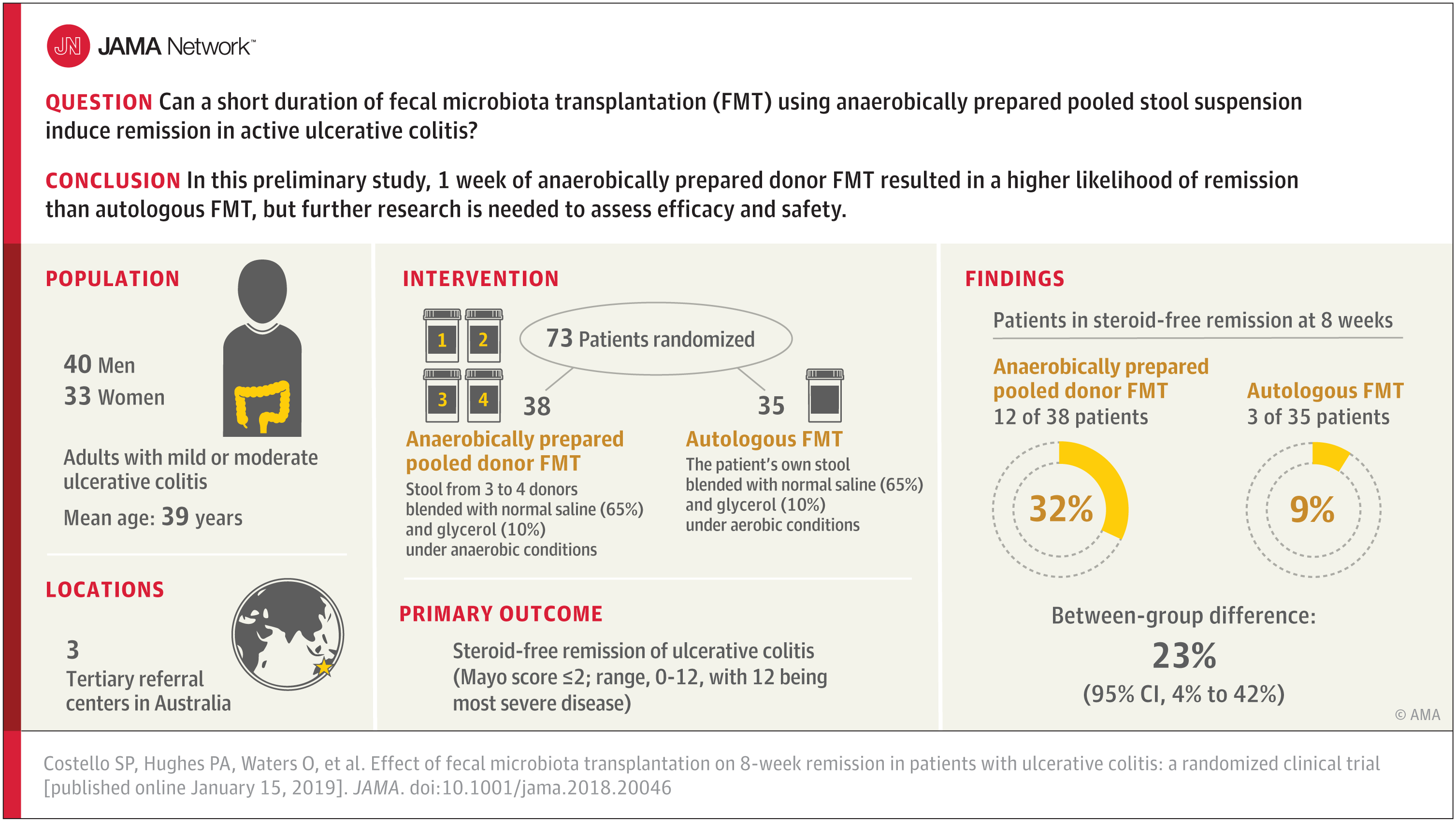
Video embed code:
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JANUARY 14, 2019
Media Advisory: To contact corresponding author Lorena Fernández de la Cruz, Ph.D., email lorena.fernandez.de.la.cruz@ki.se. The full study is available on the For The Media website.
To place an electronic embedded link in your story: Links will be live at the embargo time: https://jamanetwork.com/journals/jamaneurology/fullarticle/2719821?guestAccessKey=5b33b76d-7aac-4efd-8142-038da2293ace
Bottom Line: An observational study of people living in Sweden suggests a diagnosis of Tourette syndrome or chronic tic disorder was associated with higher risk of a cardiometabolic disorder. Among more than 14 million people living in Sweden between 1973 and 2013, about 7,800 had a registered diagnosis of Tourette syndrome or chronic tic disorder. The risk of a cardiometabolic disorder was higher than in the general population or among siblings without Tourette syndrome or chronic tic disorder. The study group doesn’t represent all Swedish patients with Tourette syndrome or chronic tic disorder because some with mild tics don’t seek care and others diagnosed by nonspecialists weren’t included.
Authors: Lorena Fernández de la Cruz, Ph.D., of the Karolinska Institutet, Stockholm, Sweden, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamaneurol.2018.4279)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JANUARY 14, 2019
Media advisory: To contact study author John W. Ayers, Ph.D., M.A., email ayers.john.w@gmail.com. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2720125?guestAccessKey=f419264c-db4b-41bb-87a3-ce20c7c487c3
Bottom Line: The national helpline 800-662-HELP is the only free, federally managed and endorsed U.S. addiction treatment referral service. This study examined public awareness of this important resource. Researchers looked at engagement and public awareness of the helpline on Google, Google News and Twitter in the week after singer Demi Lovato was hospitalized for an overdose in July 2018. They compared engagement and awareness with that of the National Suicide Prevention Lifeline (800-273-TALK) in the week after celebrity chef Anthony Bourdain’s suicide in June 2018. The table below details engagement of the two helplines. The results suggest the substance abuse helpline 800-662-HELP appears to be underappreciated in the media (they should be encouraged to include it in stories on addiction) and by the public at large (social media and internet search companies could help promote it).
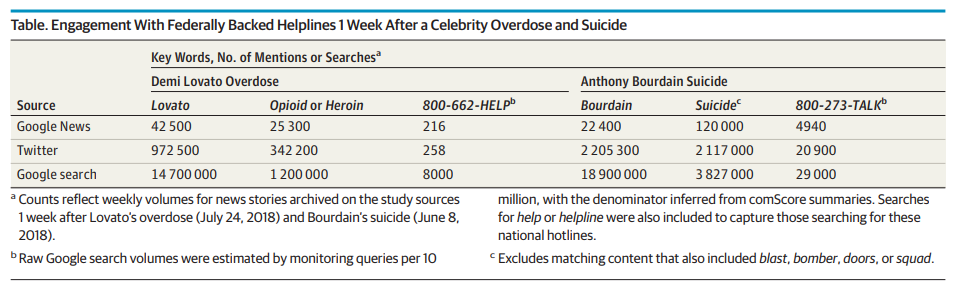
Authors: John W. Ayers, Ph.D., M.A., of the University of California, San Diego, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamainternmed.2018.6562)
Editor’s Note:Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or emailmediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JANUARY 14, 2019
Media advisory: To contact corresponding author Jaclyn Hall, Ph.D., email Douglas Bennett at dougbennett@ufl.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jamapediatrics/fullarticle/2721000?guestAccessKey=737e41bf-2442-4e6b-93a2-8e18dc2fb368
Bottom Line: Smoking during pregnancy is a leading preventable cause of adverse birth outcomes, including preterm birth, low birth weight and poor lung function. This study looked at whether change in the number of stores selling tobacco products in six Southeast states (Florida, Georgia, Mississippi, North Carolina, South Carolina and Tennessee) was associated with change in rates of smoking during pregnancy. The number of tobacco retailers in the Southeast increased by about 8,300 in 2012-2013 when Family Dollar and Dollar General started selling tobacco products; then decreased in 2014 by about 2,500 when the CVS pharmacy chain discontinued tobacco sales. Researchers found rates of smoking during pregnancy decreased 15.6 percent across the six-state region between 2011-2012 and 2015-2016 but contrasting policy changes by tobacco retailers led to an overall increase in tobacco retailer density of one additional store per 10,000 adults. Rates went down less in areas where there were more stores. Increased retail availability of tobacco products may be inhibiting progress in reducing smoking.
Author: Jaclyn Hall, Ph.D., University of Florida, Gainesville, and coauthors
Related Material: Also available on this page for listening and downloading, an interview with study coauthor Jaclyn Hall, Ph.D.
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamapediatrics.2018.4598)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, JANUARY 15, 2019
Media advisory: To contact corresponding author Richard K. Burt, M.D., email Marla Paul at marla-paul@northwestern.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time: https://jamanetwork.com/journals/jama/fullarticle/10.1001/jama.2018.18743
Bottom Line: In a randomized clinical trial, researchers compared the effect of a stem cell transplant using a non-myeloablative regimen (a lower-dose, short course of more tolerable immune specific chemotherapy and antibodies to suppress the immune system) versus continuing disease-modifying therapy in 110 patients with relapsing-remitting multiple sclerosis. The primary outcome was disease progression and other outcomes included neurologic disability, quality of life, time to relapse and no evidence of disease activity. The stem cell transplant was better than continued drug therapy for patients with frequent relapses and moderate disability. Further studies are needed to replicate the findings of this preliminary study.
Authors: Richard K. Burt, M.D., Northwestern University Feinberg School of Medicine, Chicago, and coauthors.
Visual Abstract

Related Material
The following related elements from the JAMA Network are also available on the For The Media website:
— The JAMA study, “Association of Initial Disease-Modifying Therapy With Later Conversion to Secondary Progressive Multiple Sclerosis,” by Tomas Kalincik, Ph.D., Royal Melbourne Hospital, Melbourne, Australia, and coauthors.
— The JAMA editorial, “Stem Cell Transplantation to Treat Multiple Sclerosis,” by Harold Atkins, M.D., F.R.C.P.C., of the University of Ottawa, Ottawa, Canada.
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jama.2018.18743)
Editor’s Note: The article includes funding/support and conflict of interest disclosures. Please see the article for additional information, including author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, JANUARY 9, 2019
Media advisory: To contact corresponding author Yssra S. Soliman, B.A., email Elaine Iandoli at elaine.iandoli@einstein.yu.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jamadermatology/fullarticle/10.1001/jamadermatol.2018.4813
Bottom Line: The specialty of dermatology is one of the least diverse medical fields. In this study, a survey was conducted among 155 medical students (58 percent of whom were nonwhite) to understand perceived barriers to pursuing a career in dermatology by minority medical students. Major barriers cited by minority students included the lack of diversity in dermatology; perceived negative perceptions of minority students by residency programs, such as expecting lower performance; socioeconomic factors, such as lack of loan forgiveness; and a lack of mentors. The findings highlight the need to actively recruit and mentor students of all backgrounds. The survey respondents may not be representative of all U.S. medical students.
Authors: Yssra S. Soliman, B.A., Albert Einstein College of Medicine, Bronx, New York, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamadermatol.2018.4813)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.

EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, JANUARY 8, 2019
Media advisory: To contact corresponding author Steven Woloshin, M.D., M.S., email Paige Stein at Paige.Stein@dartmouth.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time: https://jamanetwork.com/journals/jama/fullarticle/10.1001/jama.2018.19320
Bottom Line: The amount of money spent on medical marketing has increased substantially in the United States over the last two decades. An analysis estimates spending on medical marketing of drugs, disease awareness campaigns, health services and laboratory testing increased to $29.9 billion in 2016 from $17.7 billion in 1997. Most of the 2016 spending ($20.3 billion) was on marketing to professionals, while direct-to-consumer advertising grew to $9.6 billion. Regulatory oversight remains limited despite the increase in spending on marketing. This study may underestimate the amount of spending on medical marketing because some data are unavailable. The analysis was done with data and information from various sources, including the U.S. Food and Drug Administration and Centers for Medicare & Medicaid Services.
Authors: Lisa M. Schwartz, M.D., M.S., and Steven Woloshin, M.D., M.S., Dartmouth Institute for Health Policy and Clinical Practice, Lebanon, New Hampshire.
Related Material: The following are also available on the For The Media website.
The Editor’s Note, “A Tribute to Lisa M. Schwartz, M.D., M.S.,” by Howard Bauchner, M.D., Editor in Chief, JAMA.
The editorial, “Medical Marketing, Trust, and the Patient-Physician Relationship,” by Selena E. Ortiz, Ph.D., M.P.H., Pennsylvania State University, University Park, and Meredith B. Rosenthal, Ph.D., Harvard T. H. Chan School of Public Health, Boston.
The editorial, “Medical Marketing in the United States – A Truly Special Communication,” by Howard Bauchner, M.D., Editor in Chief, JAMA, and Phil B. Fontanarosa, M.D., M.B.A., Executive Editor, JAMA.
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jama.2018.19320)
Editor’s Note: The article includes conflict of interest disclosures. Please see the article for additional information, including author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JANUARY 7, 2019
Media advisory: To contact study author Hongying Dai, Ph.D., email Lisa Spellman at lspellman@unmc.edu. The full study is available on the For The Media website. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jamapediatrics/fullarticle/10.1001/jamapediatrics.2018.4595
Bottom Line: Self-reported use of flavored tobacco products by middle and high school students decreased from 2014 to 2016 but climbed back up in 2017 in an analysis of national survey data. Flavored noncigarette tobacco products are widely available in the U.S. This study examined changes in self-reported use of flavored tobacco products by youth who use tobacco. The analysis included more than 78,0000 students from a combined 2014 to 2017 national school-based annual survey. The use of any tobacco product dropped from 17.3 percent in 2014 to 13.6 percent in 2017. While the use of flavored tobacco products by those who use tobacco decreased from 69.4 percent in 2014 to 57.7 percent in 2016, it increased again to 63.6 percent between 2016-2017 and much of that appears due to flavor use in electronic cigarettes.
Authors: Hongying Dai, PhD, College of Public Health, University of Nebraska, Omaha.
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamapediatrics.2018.4595)
Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, JANUARY 4, 2019
Media advisory: To contact corresponding study author Gideon Koren, M.D., email gidiup_2000@yahoo.com. The full study is available on the For The Media website.
Want to embed a link to this study in your story?: Links will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2018.6643
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: Holocaust survivors had higher rates of chronic conditions but lower rates of death than a comparison group of individuals insured by the same healthcare services organization in Israel. Biological and psychosocial reasons that may help to explain the findings need more study but researchers suggest unique characteristics of resilience among Holocaust survivors and better health literacy may be among the possibilities. This observational study included more than 38,000 Holocaust survivors in Israel who were born between 1911 and 1945 in Europe and nearly 35,000 people in a control group born in Israel during those same years. Both groups were insured by Maccabi Healthcare Services in Israel. The study used data collected from 1998 through 2017 and looked at heart disease, chronic kidney disease, chronic obstructive pulmonary disease, osteoporosis, diabetes, hypertension, cancer and death.
Authors: Gideon Koren, M.D., of Maccabi Healthcare Services, Tel Aviv, Israel, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamanetworkopen.2018.6643)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, JANUARY 4, 2019
Media advisory: To contact corresponding author Jakob Christensen, M.D., Ph.D., Dr.Med.Sci., email jakob@farm.au.dk. The full study is available on the For The Media website.
Want to embed a link to this study in your story?: Link will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2018.6606
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: This study examined whether prenatal exposure to valproate and other antiepileptic drugs was associated with increased risk of attention-deficit/hyperactivity disorder (ADHD) in children. More than 913,000 children in Denmark were included in the observational study, and exposure to antiepileptic drugs was defined as pregnancies where mothers redeemed one or more prescriptions for the medications. In total, 580 children were identified as having been exposed to valproate during pregnancy and, of them, 49 (8.4 percent) had ADHD; among more than 912,000 children not exposed to valproate about 29,000 (3.2 percent) had ADHD. The study used registry data and it is not known whether the women used the medication and how much was actually taken. The absolute 15-year risk of ADHD in children exposed to valproate in pregnancy was higher than those not exposed to the drug. There were no associations found between other antiepileptic drugs in the study and ADHD.
Authors: Jakob Christensen, M.D., Ph.D., Dr.Med.Sci., Aarhus University Hospital, Aarhus, Denmark, and coauthors
Related Material: The commentary, “Fetal Valproate Exposure and Attention-Deficit/Hyperactivity Disorder,” by Kimford J. Meador, M.D., Stanford University School of Medicine, Palo Alto, California, is also available on the For The Media website.
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamanetworkopen.2018.6606)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or emailmediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, DECEMBER 28, 2018
Media advisory: To contact corresponding study author Richard E. Tremblay, Ph.D., email richard.ernest.tremblay@umontreal.ca. The full study is available on the For The Media website.
Want to embed a link to this study in your story?: Links will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2018.6364
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: Children can exhibit physical aggression when they are very young but that behavior typically declines before and during elementary school. However, a small proportion of children have atypically high physical aggression problems into adolescence, which may put them at increased risk for violent crime, social maladjustment, and alcohol and drug abuse. This observational study of 2,223 boys and girls used information from mothers, teachers and the children to trace the development of physical aggression problems from infancy to adolescence. The analysis suggests the frequency of physical aggression increased from age 1½ to 3½ and then decreased until age 13. Trajectories for the development of physical aggression differed for boys and girls, and several risk factors were identified, including family characteristics when the child was an infant such as having parents with lower education and higher depression, lower socioeconomic status and a higher number of siblings. Interventions during pregnancy and early childhood may help to prevent high physical aggression in children in high-risk families.
Authors: Richard E. Tremblay, Ph.D., University of Montreal, Montreal, Quebec, Canada, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamanetworkopen.2018.6364)
Editor’s Note: The article contains funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, DECEMBER 28, 2018
Media Advisory: To contact corresponding author Kimberley J. Smith, Ph.D., email kimberley.j.smith@surrey.ac.uk. The full study is available on the For The Media website.
To place an electronic embedded link in your story: Links will be live at the embargo time: https://jamanetwork.com/journals/jamaneurology/fullarticle/10.1001/jamaneurol.2018.4147
Bottom Line: While cerebral palsy is considered a pediatric condition because it develops and is diagnosed in early childhood, it is a lifelong condition with the majority of children living into adulthood. Little research exists on the mental health of adults with cerebral palsy. This study included 1,700 adults 18 years or older with cerebral palsy and 5,100 adults without cerebral palsy. Those adults with cerebral palsy without an intellectual disability had a higher risk of developing depression and anxiety. The study relied on diagnostic codes for outcomes.
Authors: Kimberley J. Smith, Ph.D., University of Surrey, Guildford, United Kingdom, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamaneurol.2018.4147)
Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, DECEMBER 28, 2018
Media advisory: To contact corresponding study author Alexander J. Butwick, M.B.B.S., F.R.C.A., M.S., email Erin Digitale at digitale@stanford.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story?: Links will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2018.6567
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: Pain relief for pregnant women in labor is commonly given in the form of epidural, spinal or combined spinal-epidural blockade, which is collectively referred to as neuraxial analgesia. This study used birth certificate data and found wide variation in neuraxial analgesia use across the United States. Among 2.6 million pregnant women who underwent labor in 2015, neuraxial analgesia was used by 73 percent, with the lowest frequency in Maine and the highest in Nevada. Variation between states was only partly explained by state-level factors, which suggests other unmeasured patient-level and hospital-level factors likely were at play. It’s important to understand the main reasons behind the variation and to know whether it influences health outcomes for women and newborns.
Authors: Alexander J. Butwick, M.B.B.S., F.R.C.A., M.S., Stanford University School of Medicine, Stanford, California, and coauthors
Visual Abstract:

To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamanetworkopen.2018.6567)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), THURSDAY, DECEMBER 27, 2018
Media advisory: To contact corresponding author Farhad lslami, M.D., Ph.D., email David Sampson at david.sampson@cancer.org. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jamaoncology/fullarticle/10.1001/jamaoncol.2018.5639
Bottom Line: Extra body weight is associated with cancer. This study used data from several sources to examine cancer cases attributed to excess weight among adults 30 or older in 50 states and the District of Columbia. Each year from 2011 to 2015, an estimated 38,000 cases of cancer in men and 75,000 in women were attributed to extra body weight. That excess weight accounted for at least 1 in 17 of all new cancers in each state. The proportion of cancer cases varied among states, with the highest proportions found in several Southern and Midwestern states, Alaska and the District of Columbia. The study has some limitations, including that proportions for some cancer types in less populated states are based on a small number of cases.
Authors: Farhad lslami, M.D., Ph.D., American Cancer Society, Atlanta, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamaoncol.2018.5639)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, DECEMBER 28, 2018
Media advisory: To contact study author Adil Haider, M.D., M.P.H., email Johanna Younghans at jyounghans@bwh.harvard.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story?: Links will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2018.6506
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: Emergency department patients who are sexual or gender minorities reported greater satisfaction when information on sexual and gender identity was collected on forms during registration instead of by nurses who asked about it during the visit. Understanding patient preferences for collecting this information is important because health care disparities exist for sexual and gender minority patients (including lesbian, gay, bisexual or transgender identities) but the extent of those disparities is not known because of a lack of routine collection of information about sexual and gender identity from patients. This observational study analyzed survey data from 540 emergency department. It is unclear if these findings can be generalized to medical settings outside the emergency department.
Authors: Adil Haider, M.D., M.P.H., Brigham & Women’s Hospital, Harvard Medical School, Boston, Massachusetts, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamanetworkopen.2018.6506)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or emailmediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, DECEMBER 28, 2018
Media advisory: To contact study author David L. Brown, M.D., email Diane Duke Williams at Williamsdia@wustl.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story?: Links will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2018.6383
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: Having seven or fewer alcoholic drinks a week was associated with increased survival in older adults with newly diagnosed heart failure compared with patients who abstained from alcohol after accounting for other potential mitigating factors. Conflicting data exist about an association between alcohol consumption and heart failure but not much is known about the safety of alcohol consumption in patients after a new diagnosis of heart failure. This observational study of 393 patients suggests limited alcohol consumption of seven drinks a week or fewer was associated with an additional average survival of just over one year at 383 days compared with abstinence from alcohol. Survival after a new diagnosis of heart failure was about 7.5 years among patients in the study. Researchers didn’t have information about the cause of heart failure in these patients. Optimal levels of alcohol consumption by adults with heart failure remain to be determined. These results should not be interpreted as suggesting that individuals with newly diagnosed heart failure show begin drinking alcohol after their diagnosis if they did not drink previously.
Authors: David L. Brown, M.D., Washington University School of Medicine, St. Louis, Missouri, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamanetworkopen.2018.6383)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or emailmediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, DECEMBER 28, 2018
Media advisory: To contact corresponding author Julie R. Gaither, Ph.D., M.P.H., R.N., email julie.gaither@yale.edu or Ziba Kashef at Ziba.kashef@yale.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story?: Links will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2018.6558
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: Nearly 9,000 children and adolescents died from opioid poisonings with prescription and illicit drugs between 1999 and 2016 based on an analysis of national data. The death rate almost tripled over that time to nearly 1 per 100,000 based on the data from the Centers for Disease Control and Prevention (CDC). Prescription opioids were implicated in 73 percent of the deaths (6,561) and most of the deaths were unintentional (nearly 81 percent). The majority of deaths were among non-Hispanic white males but over time non-Hispanic black children accounted for a larger proportion of the deaths. The highest annual death rates during the 18 years examined in the study were among teens 15 to 19, with heroin implicated in nearly 1,900 deaths. The study relied on data from death certificates so the potential for misclassification of cause and manner of death exists. Researchers urge lawmakers, public health officials, clinicians and parents to implement protective measures to address the growing public health problem.
Authors: Julie R. Gaither, Ph.D., M.P.H., R.N., Yale School of Medicine, New Haven, Connecticut, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamanetworkopen.2018.6558)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or emailmediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, DECEMBER 21, 2018
Media advisory: To contact corresponding author Lucio C. Rovati, M.D., email lucio.rovati@unimib.it. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time: https://jamanetwork.com/journals/jama/fullarticle/10.1001/jama.2018.19319
Bottom Line: Managing osteoarthritis requires long-term treatment for symptoms such as pain and changes in joint structure that can lead to disability. This study analyzed the combined results of 47 randomized clinical trials that lasted at least 12 months for 33 drug interventions and 22,000 patients with knee osteoarthritis. Researchers report uncertainty around the long-term effectiveness of medications to control pain for patients with knee osteoarthritis, including the two medications that were associated with improved pain (celecoxib and glucosamine sulfate). Large randomized clinical trials are needed to resolve questions regarding long-term pain control.
Authors: Lucio C. Rovati, M.D., University of Milano – Bicocca, Monza, Italy, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jama.2018.19319)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, DECEMBER 21, 2018
Media advisory: To contact study author John W. Ayers, Ph.D., M.A., email ayers.john.w@gmail.com. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Links will be live at the embargo time https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/10.1001/jamainternmed.2018.5094
Bottom Line: An estimated 40 to 54 million Google searches for sexual harassment and assault were recorded in the United States in the eight months after public accusations against film producer Harvey Weinstein and the ensuing #MeToo movement. Searches related to reporting and preventing such actions also were up based on the results of a study that monitored and analyzed search activity.
Authors: John W. Ayers, Ph.D., M.A., of the University of California, San Diego, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamainternmed.2018.5094)
Editor’s Note: The article contains funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or emailmediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, DECEMBER 21, 2018
Media advisory: To contact study author Oanh Kieu Nguyen, M.D., M.A.S., email Peter Farley at Peter.Farley@ucsf.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Links will be live at the embargo time https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/10.1001/jamainternmed.2018.5866
Bottom Line: A unique opportunity made it feasible for uninsured patients with end-stage renal disease (ESRD) who received emergency-only dialysis in Dallas, Texas, to enroll in private, commercial health insurance plans in 2015 and that made it possible for researchers to compare scheduled vs. emergency-only dialysis among undocumented immigrants with ESRD. This observational study included 181 undocumented immigrants, 105 of whom received insurance coverage and enrolled in scheduled dialysis and 76 of whom remained uninsured. Regularly scheduled dialysis (the standard of care for ESRD) compared with emergency-only dialysis (administered when a patient becomes life-threateningly ill) was associated with reductions in mortality, health care utilization and costs among patients with ESRD. The authors call for scheduled dialysis to be the standard of care for any patient with ESRD in the United States.
Authors: Oanh Kieu Nguyen, M.D., M.A.S., of the University of California, San Francisco, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamainternmed.2018.5866)
Editor’s Note: The article contains funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or emailmediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, DECEMBER 21, 2018
Media advisory: To contact corresponding author Robert W. Yeh, M.D., M.Sc., email Lindsey Diaz-MacInnis at ldiaz2@bidmc.harvard.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time: https://jamanetwork.com/journals/jama/fullarticle/10.1001/jama.2018.19232
Bottom Line: The Hospital Readmissions Reduction Program (HRRP) was created under the Affordable Care Act and hospitals face financial penalties for higher-than-expected 30-day readmission rates for patients with heart failure, heart attack and pneumonia. Lower hospital readmission rates for those conditions have been associated with the program but it was unclear if the program was associated with a change in patient deaths. This observational study included 8 million Medicare hospitalizations for heart failure, heart attack and pneumonia before and after HRRP was implemented. Study results suggest implementation of the HRRP was associated with an increase in deaths within 30 days after discharge for hospitalization for heart failure and pneumonia but not for heart attack. More research is needed to understand if the increase in 30-day postdischarge mortality is a result of the program, considering a lack of association with mortality within 45 days of hospital admission.
Authors: Robert W. Yeh, M.D., M.Sc., Beth Israel Deaconess Medical Center, Boston, and coauthors
Related Material: The editorial, “Unintended Harm Associated With the Hospital Readmissions Reduction Program,” by Gregg C. Fonarow, M.D., Ronald Reagan UCLA Medical Center, Los Angeles, is also available on the For The Media website.
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jama.2018.19232)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
FOR IMMEDIATE RELEASE: DECEMBER 19, 2018
Media advisory: To contact corresponding author Julia A. Dilley, Ph.D., M.E.S., email Kate Willson at kate.willson@multco.us. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jamapediatrics/fullarticle/10.1001/jamapediatrics.2018.4458
Bottom Line: Washington state legalized retail marijuana for adults 21 and older in 2012. Understanding how this change affects teens is important. This study assessed whether findings about marijuana use by young people in a Washington state-based youth survey were consistent with findings from a nationally representative survey. An analysis of the two surveys of students in the eighth, 10th and 12th grades finds differing results on whether marijuana use has increased or decreased among teens. The results of one survey suggest use declined after legalization among eighth and 10th graders; the other survey suggests an increase in use among 10th graders. Neither survey showed changes in use among 12th graders. Differences between the surveys in design and methods may explain the findings.
Author: Julia A. Dilley, Ph.D., M.E.S., Multnomah County/Oregon Public Health Division, Portland, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamapediatrics.2018.4458)
Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, DECEMBER 21, 2018
Media advisory: To contact corresponding study author Daniel M. Tomaszewski, Pharm.D., Ph.D., email Bethanie Le at bele@chapman.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story?: Links will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2018.6161
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: Opioids for pain management in pediatric patients are sometimes necessary but their use has raised concerns about the effects of opioids and later abuse. This analysis examined opioid prescribing rates using information from the National Hospital Ambulatory Medical Care Survey from 2006 to 2015 on more than 69,000 emergency department visits for patients younger than 18. Prescribing rates decreased from 8.2 percent in 2006–2010 to 6.3 percent in 2011–2015. Prescribing seemed to vary by region of the country, race, age and payment. For example, opioid prescribing rates were higher in the West; white patients and patients 13 to 17 were more likely to get prescriptions; and patients using Medicaid were less likely to get opioid prescriptions. The results of this observational study suggest inconsistencies in opioid prescribing requiring further research.
Authors: Daniel M. Tomaszewski, Pharm.D., Ph.D., Chapman University, Irvine, California, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamanetworkopen.2018.6161)
Editor’s Note: The article contains funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
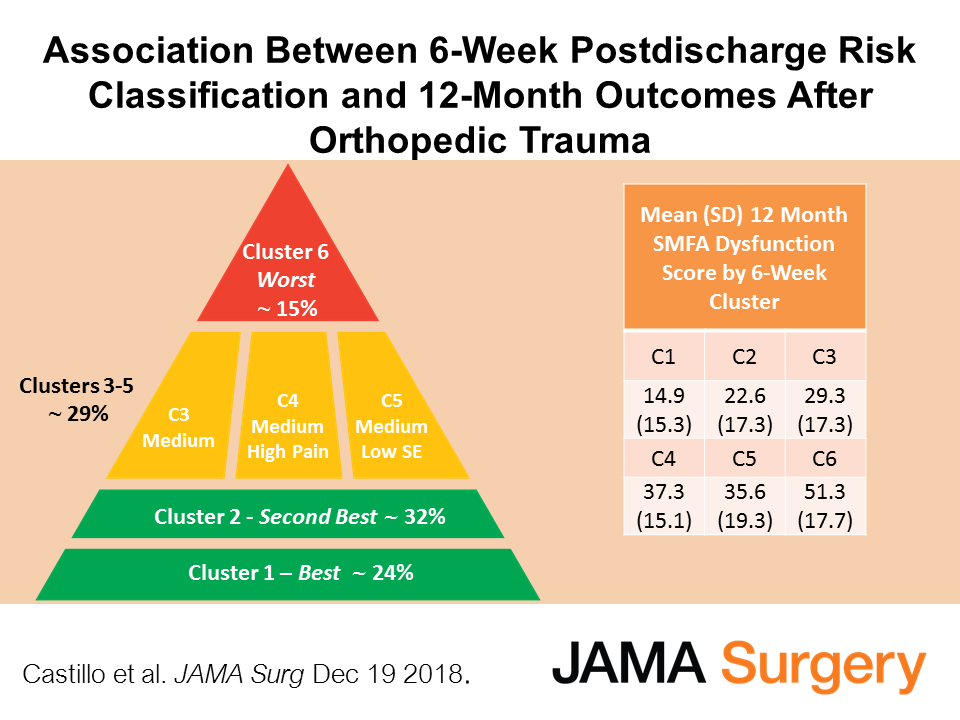
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, DECEMBER 21, 2018
Media advisory: To contact corresponding study author Eli S. Rosenberg, Ph.D., email Kelsey Butz at kebutz@albany.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story?: Links will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2018.6371
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: Hepatitis C virus infection is a major cause of illness and death in the United States and injection drug use is likely fueling many new cases. This study, which used survey and vital statistics data, suggests about 1 percent of adults (0.93 percent) were living with hepatitis C from 2013 to 2016, and how common infections were varied by state and region. Nine states accounted for about 52 percent of all people living with hepatitis C (California, Texas, Florida, New York, Pennsylvania, Ohio, Michigan, Tennessee and North Carolina). The highest rates of infection were frequently in states heavily impacted by the opioid crisis, with 5 of 9 states with the highest number of hepatitis C infections in the Appalachian region. The results of this observational study could help to guide state-level prevention and treatment efforts because the resources necessary will vary by jurisdiction.
Authors: Eli S. Rosenberg, Ph.D., University at Albany School of Public Health, State University of New York, Rensselaer, New York, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamanetworkopen.2018.6371)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Listen to an interview with Robert A. Tessler, M.D., M.P.H., and Frederick P Rivara, M.D., M.P.H., co-authors of the JAMA Surgery study, “Trends in Firearm Injury and Motor Vehicle Crash Case Fatality by Age Group, 2003-2013.” The podcast is available for listening and download on this page.
Visual Abstract
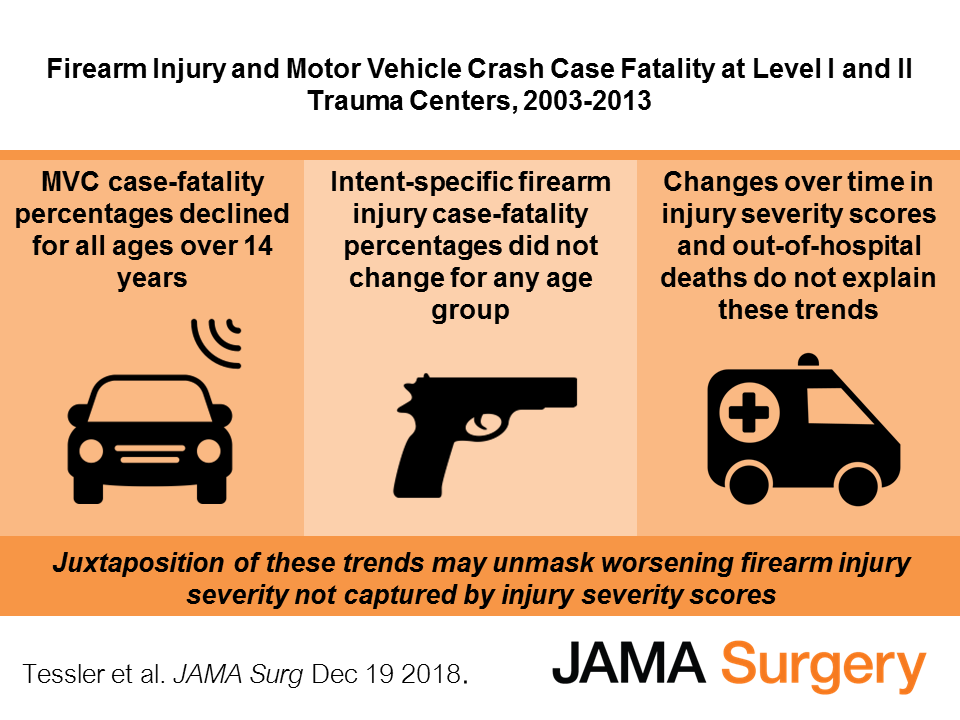
EMBARGOED FOR RELEASE: 11 A.M. (ET), THURSDAY, DECEMBER 20, 2018
Media advisory: To contact corresponding author Keisuke Kawata, Ph.D., email April Toler at artoler@iu.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jamaophthalmology/fullarticle/10.1001/jamaophthalmol.2018.6193
Bottom Line: Head impacts in youth sports, even when they don’t cause symptoms of concussion, are a public health concern because these so-called subconcussive head impacts may result in long-term neurological issues if they are sustained repeatedly. This study looked at changes in measurements of near point of convergence (NPC), which is the distance from your eyes to where both eyes can focus without double vision, in 12 high school football players at 14 different times during a season. The NPC measurement matters because it has been shown to detect damage to neurons before symptoms appear. The frequency and magnitude of head impacts from all practices and games also were measured. Study findings suggest NPC values worsened with subconcussive head impacts, and that impaired NPC didn’t rapidly recover. However, NPC values began to return to normal in midseason while players continued to incur head impacts, suggesting the system controlling eye movements may develop tolerance to recurrent subconcussive head impacts. The findings of this study may not be generalized because of its small size.
Author: Keisuke Kawata, Ph.D., Indiana University, Bloomington, and coauthors
Related Material: The commentary, “Assessing Subconcussive Head Impacts in Athletes Playing Contact Sports – The Eyes Have It,” by Ann C. McKee, M.D., and Michael L. Alosco, Ph.D., of the Boston University School of Medicine, is also available on the For The Media website.
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamaophthalmol.2018.6193)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, DECEMBER 17, 2018
Media advisory: To contact corresponding author Melissa A. Bright, Ph.D., email mbright@coe.ufl.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jamapediatrics/fullarticle/10.1001/jamapediatrics.2018.4346
Bottom Line: This study used a complex method to analyze report card release dates and cases of child physical abuse called into a hotline and verified by Florida’s child welfare agency for elementary school children during an academic year. In an analysis that included 1,943 cases of verified child physical abuse, calls that resulted in verified cases came in at a higher rate on Saturdays when report cards were released on Fridays. Possible reasons to explain why are speculative and require further study. The study is limited by its focus only on public school data and data only on physical abuse that resulted in calls to a state hotline.
Authors: Melissa A. Bright, Ph.D., of the University of Florida, Gainesville, and coauthors
Related Material: The editorial, “Corporal Punishment and Children’s Report Cards – Failing Our Children,” by Antoinette L. Laskey, M.D., M.P.H., M.B.A., of the University of Utah, Salt Lake City, also is available on the For The Media website.
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamapediatrics.2018.4346)
Editor’s Note: The article contains funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, DECEMBER 18, 2018
Media advisory: To contact corresponding author Jason W. Busse, D.C., Ph.D., email Veronica McGuire at vmcguir@mcmaster.ca. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time: https://jamanetwork.com/journals/jama/fullarticle/10.1001/jama.2018.18472
Bottom Line: An estimated 50 million adults in the United States were living with chronic noncancer pain in 2016 and many of them were prescribed opioid medications, even though a clinical benefit is uncertain. This study combined the results of 96 randomized clinical trials with about 26,000 participants to compare opioids with placebo and nonopioid alternative pain medications for the treatment of chronic noncancer pain. Opioids were associated with small improvements in pain and physical functioning plus increased risk of vomiting compared with placebo. Comparisons of opioids with nonopioid pain medication alternatives suggest the benefit for pain and functioning may be similar but the quality of evidence from the studies wasn’t high. None of the studies provided rates of developing opioid use disorder.
Authors: Jason W. Busse, D.C., Ph.D., McMaster University, Hamilton, Ontario, Canada, and coauthors
Related Material
The following related elements from the JAMA Network are also available on the For The Media website:
A summary video for this study is available to view on this page and to embed on your website by copying and pasting the HTML code below. To download the video, email mediarelations@jamanetwork.org for information.
The JAMA editorial, “Increasing Evidence for the Limited Role of Opioids to Treat Chronic Noncancer Pain,” by Michael A. Ashburn, M.D., M.P.H., and Lee A. Fleisher, M.D., of the University of Pennsylvania, Philadelphia.
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jama.2018.18472)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Video embed code:

EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, DECEMBER 17, 2018
Media Advisory: To contact corresponding author Guy Fagherazzi, Ph.D., email guy.fagherazzi@gustaveroussy.fr. The full study is available on the For The Media website.
To place an electronic embedded link in your story: Links will be live at the embargo time: https://jamanetwork.com/journals/jamaneurology/fullarticle/10.1001/jamaneurol.2018.3960
Bottom Line: A study of French women suggests a lower risk of type 2 diabetes was observed among women who reported current migraines compared with women with no history of the painful headaches.
Why The Research Is Interesting: Migraine and type 2 diabetes are common conditions in women but an association between them remains unclear.
Who and What: A group of more 74,000 French women insured by a health plan that mostly covered teachers and who were followed up by questionnaire.
How (Study Design): This was an observational study. Researchers didn’t intervene for purposes of the study and they cannot control all the factors that could explain the study findings.
Authors: Guy Fagherazzi, Ph.D., of the Center for Research in Epidemiology and Population Health, Villejuif, France, and coauthors
Results: 
Study Limitations: Potential reasons that could explain the observations by researchers are uncertain.
Study Conclusions: The results of this study could have implications on the understanding of the underlying causes of these two common conditions and more research is needed to understand potential reasons that could explain these findings.
Related Material: The editorial, “Potential Benefits of Migraine – What Is It Good For?” by Amy A. Gelfand, M.D., M.A.S., of the University of California, San Francisco, and a JAMA Neurology associate editor, and Elizabeth Loder, M.D., M.P.H., of Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, also is available on the For The Media website.
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamaneurol.2018.3960)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), THURSDAY, DECEMBER 13, 2018
Media advisory: To contact corresponding author Michael V. Boland, M.D., email Jianyi Nie at jnie4@jhmi.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jamaophthalmology/fullarticle/10.1001/jamaophthalmol.2018.6066
Bottom Line: Mobile device reminders have been associated with better medication adherence and linking reminders to patient electronic health records (EHRs) could potentially allow some oversight by clinicians. In this study, 100 patients (average age 65) agreed to set up electronic health record-linked reminders delivered via text or voice message for glaucoma medications for three months and were surveyed about the experience. Of the participants, 94 ultimately set up reminders and 89 completed follow-up. Of these patients, 74 percent reported the reminders were useful, 15 percent were neutral about them and 11 percent found them not useful. Most participants (81 percent) had help setting up reminders. The generalizability of these results and the effect on glaucoma outcomes remains unknown.
Author: Michael V. Boland, M.D., Johns Hopkins University School of Medicine, Baltimore, and coauthors
Related Material: The commentary, “Preliminary Steps to Address Glaucoma Medication Adherence,” by Paula Anne Newman-Casey, M.D., M.S., University of Michigan, Ann Arbor, and Jonathan S. Myers, M.D., Thomas Jefferson University, Philadelphia, is also available on the For The Media website.
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamaophthalmol.2018.6066)
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, DECEMBER 18, 2018
Media advisory: To contact corresponding author Joan L. Blomquist, M.D., email John Lazarou at jlazarou@gbmc.org. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time: https://jamanetwork.com/journals/jama/fullarticle/10.1001/jama.2018.18315
Bottom Line: Pelvic floor disorders such as urinary incontinence and pelvic organ prolapse (when one or more of the pelvic organs drop from their normal position) are associated with childbirth and affect millions of women in the United States. This study examined the risk of pelvic floor disorders based on the method of childbirth delivery among 1,500 women a decade or two after giving birth. Cesarean delivery compared with spontaneous vaginal delivery was associated with less risk of stress urinary incontinence, overactive bladder and pelvic organ prolapse. An operative vaginal delivery, such as using forceps or one that is vacuum-assisted, was associated with higher risk of anal incontinence and pelvic organ prolapse. The data for the study were from a single hospital so the results may not be generalized to all populations.
Authors: Joan L. Blomquist, M.D., Greater Baltimore Medical Center, Maryland, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jama.2018.18315)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, DECEMBER 12, 2018
Media advisory: To contact corresponding author Wayne A. Ray, Ph.D., email Craig Boerner at craig.boerner@vumc.org. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jamapsychiatry/fullarticle/10.1001/jamapsychiatry.2018.3421
Bottom Line: Antipsychotic medications can have adverse effects, including those that are life-threatening. This observational study examined the association of antipsychotic medications prescribed for children and young adults without psychosis and risk of unexpected death, which includes deaths due to unintentional drug overdose or cardiovascular/metabolic causes. About 250,000 children and young people (ages 5 to 24) enrolled in Medicaid in Tennessee were included. They were new users of antipsychotic medications who received higher or lower doses and new users of control medications that weren’t antipsychotics for comparison. An increased risk of unexpected death was associated with the group of patients who received a higher dose of antipsychotic medication compared with those who didn’t. Other factors could explain the differences between users of antipsychotics and control medications. These findings appear to reinforce careful prescribing and monitoring of antipsychotic medications in children and young people.
Authors: Wayne A. Ray, Ph.D., Vanderbilt University School of Medicine, Nashville, Tennessee, and coauthors
Related Material: The editorial, “Antipsychotics, Excess Deaths, and Paradoxes of Child Psychiatry,” by Barbara Geller, M.D., Washington University in St. Louis, also is available on the For The Media website.
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/ jamapsychiatry.2018.3421)
Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, DECEMBER 14, 2018
Media advisory: To contact corresponding study author Eric Sun, M.D., Ph.D., email Amy Jeter Hansen at ajeterhansen@stanford.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story?: Links will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2018.5909
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: The use of early physical therapy in a study of nearly 89,000 U.S. adults with musculoskeletal pain of the shoulder, neck, knee and low back was associated with a lower likelihood of subsequent opioid use in an analysis of health insurance claims from 2007 to 2015. For patients who did use opioids, early physical therapy was associated with reduced opioid use for shoulder, knee and low back pain but not neck pain. The findings suggest early physical therapy may play a role in reducing the risk of subsequent long-term opioid use by patients with musculoskeletal pain. This was an observational study so other potential mitigating factors could help to explain the results.
Authors: Eric Sun, M.D., Ph.D., Stanford University School of Medicine, Stanford, California, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamanetworkopen.2018.5909)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, DECEMBER 14, 2018
Media advisory: To contact corresponding study author Alice Chen, Ph.D., M.B.A., M.Sc., email Jenesse Miller at jenessem@usc.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story?: Links will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2018.5805
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: This study examined the characteristics of physicians excluded from Medicare and state public insurance programs for fraud, health care crimes or unlawful prescribing of controlled substances. There were 2,222 physicians (0.3 percent) excluded temporarily or permanently between 2007 and 2017 based on federal data. Exclusion rates were highest in the West and Southeast, with West Virginia having the highest exclusion rate at almost 6 per 1,000 physicians (32 exclusions among 5,720 physicians). Overall, physicians were more likely to be excluded if they were male, had osteopathic training, were older or practiced in specific specialties (family medicine, psychiatry, internal medicine, anesthesiology, surgery and obstetrics/gynecology). The study design prevents causal inferences but may help to identify characteristics associated with physicians more or less likely to engage in fraud.
Authors: Alice Chen, Ph.D., M.B.A., M.Sc., University of Southern California, Los Angeles, California, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamanetworkopen.2018.5850)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, DECEMBER 10, 2018
Media Advisory: To contact corresponding author Virginia J. Howard, Ph.D., email Holly Gainer at hgainer@uab.edu. The full study is available on the For The Media website.
To place an electronic embedded link in your story: Links will be live at the embargo time: https://jamanetwork.com/journals/jamaneurology/fullarticle/10.1001/jamaneurol.2018.3862
Bottom Line: This study examined the risk of stroke by sex among more than 25,000 black and white women and men. White women had lower risk of stroke than white men and black women had lower risk than black men between the ages of 45 and 64; from 65 to 74 white women still had lower risk than white men but that difference didn’t persist among black women and men; and there was no difference by sex for either race at age 75 and older. The association of some risk factors with stroke risk also differed by sex for white women and men but not for black women and men. Study participants may not be representative of the general population. The findings suggest earlier and more aggressive management of risk factors may be warranted in some demographic groups to try to prevent stroke.
Authors: Virginia J. Howard, Ph.D., of the University of Alabama at Birmingham, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamaneurol.2018.3862)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, DECEMBER 12, 2018
Media advisory: To contact corresponding author April W. Armstrong, M.D., M.P.H., email Cynthia Smith at Cynthia.Smith@med.usc.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jamadermatology/fullarticle/10.1001/jamadermatol.2018.4566
Bottom Line: Eczema (atopic dermatitis) is a chronic inflammatory skin disease that affects millions of adults and children and has been associated with depression and anxiety. Evidence on the association between eczema and suicidal thoughts or attempts has been inconclusive. This study evaluated the association between eczema and suicidal thoughts and attempts by analyzing the combined results of 15 studies including 310,000 patients with eczema and 4.4 million people without eczema. The findings suggest patients with eczema were more likely to have suicidal thoughts and attempts compared to people without eczema. Data on completed suicides were limited and had inconsistent results.
Authors: April W. Armstrong, M.D., M.P.H., University of Southern California Keck School of Medicine, Los Angeles, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamadermatol.2018.4566)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, DECEMBER 10, 2018
Media advisory: To contact corresponding author Meghan Miller, Ph.D., email Dorsey Griffith at dgriffith@ucdavis.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jamapediatrics/fullarticle/10.1001/jamapediatrics.2018.4076
Bottom Line: Siblings born in a family after other children with autism spectrum disorder (ASD) or attention-deficit/hyperactivity disorder (ADHD) were more likely to be diagnosed with the same disorder or the other disorder.
Why The Research Is Interesting: ADHD and ASD are common neurodevelopmental disorders that likely share some genetic factors and biological influences. Estimating recurrence risk in families is a way to measure shared genetic factors. Such risk estimates are often based on the total number of siblings in a family rather than being limited to later-born siblings (those born after children with ASD or ADHD) so that risk can be underestimated if families decide to stop having children after a child develops ASD or ADHD. This study focused on risk for later-born siblings.
Who and What: A total of 15,175 later-born siblings classified by familial risk based on an older child’s diagnosis: ADHD risk (730), ASD risk (158) and no known risk (14,287); data were extracted from two large health care system in the United States.
How (Study Design): This was a population-based study.
Authors: Meghan Miller, Ph.D., of the University of California Davis Health System, Sacramento, California, and coauthors
Results:

Study Limitations: These include a selective sample, lack of information on half- or full-sibling status, and data drawn from general medical records.
Study Conclusions:

Related Material: The editorial, “Later Sibling Recurrence of Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder: Clinical and Mechanistic Insights,” by Tony Charman, Ph.D., of King’s College London, and Emily J.H. Jones, Ph.D., of the University of London, both in the United Kingdom, also is available on the For The Media website.
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamapediatrics.2018.4076)
Editor’s Note: The article contains funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, DECEMBER 12, 2018
Media advisory: To contact corresponding author Heather Yeo, M.D., M.H.S., email Krystle Lopez at krl2003@med.cornell.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jamasurgery/fullarticle/10.1001/jamasurg.2018.4650
Bottom Line: Most patients who qualify for weight loss surgery don’t have the procedure despite its safety and effectiveness. One reason may be negative public attitudes toward weight loss surgery. This study assessed attitudes toward weight loss surgery with a national survey that included about 950 respondents. Nearly half reported they thought most people who had weight loss surgery did it for cosmetic reasons and about 40 percent thought people who had weight loss surgery chose the “easy way out.” Women were more likely to think most weight loss surgical procedures were performed for health reasons and less likely to think of surgery as an easy way out. The association between more negative attitudes about weight loss surgery and people not opting for it supports the suggestion that public attitudes may be at least partly responsible for the low use of weight loss surgical procedures.
Authors: Heather Yeo, M.D., M.H.S., New York-Presbyterian Hospital, Weill Cornell Medicine, New York, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamasurg.2018.4650)
Editor’s Note: Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, DECEMBER 7, 2018
Media advisory: To contact corresponding study author Emese Zsiros, M.D., Ph.D., email Annie Deck-Miller at annie.deck-miller@roswellpark.org. The full study is available on the For The Media website.
Want to embed a link to this study in your story?: Links will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2018.5452
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: An ultrarestrictive opioid prescribing strategy was associated with a reduction in the number of pills dispensed in a study of patients having surgery for gynecologic cancer, without changes in postoperative pain scores, complications or increases in prescription refill requests. Under the protocol, patients having ambulatory or minimally invasive surgery weren’t prescribed opioids at discharge unless they required more than five doses of oral or intravenous opioids while in the hospital. Surgical patients who had an abdominal incision (laparotomy) were given a three-day supply of opioids when they were discharged. The average number of opioid pills dispensed at discharge decreased after the ultrarestrictive prescribing protocol was implemented from 43.6 to 12.1 for patients who had a laparotomy; from 38.4 to 1.3 for patients who had minimally invasive surgery; and from 13.9 to 0.2 for patients who had ambulatory surgery. The findings reveal a promising strategy for decreasing postoperative opioid prescribing without increasing pain.
Authors: Emese Zsiros, M.D., Ph.D., Roswell Park Comprehensive Cancer Center, Buffalo, New York, and coauthors
Related Material: The invited commentary, “Striving for Evidence-Based Postoperative Opioid Prescribing While Optimizing Perioperative Pain Management—Shifting to Conservative Prescribing,” by Jennifer M. Hah, M.D., M.S., Stanford University, Palo Alto, California, also is available on the For The Media website.
Visual Abstract:

To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamanetworkopen.2018.5452)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, JANUARY 4, 2019
Media advisory: To contact corresponding study author Ruchi S. Gupta, M.D., M.P.H., email Vita Lerman at VLerman@luriechildrens.org. The full study is available on the For The Media website.
Want to embed a link to this study in your story?: Links will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2018.5630
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: Survey data suggest at least 1 in 10 U.S. adults are food allergic and nearly 1 in 5 believe they have a food allergy. Food allergies are expensive and potentially life-threatening conditions. In this nationally representative survey study of more than 40,000 U.S. adults, nearly half of food-allergic adults developed allergies during adulthood, many reported being allergic to multiple foods, and 38 percent reported at least one food allergy–related emergency department visit in their lifetime. Shellfish allergy was the most common, followed by milk, peanut, tree nut and fin fish. Self-reported food allergies by study participants weren’t confirmed by diagnosis.
Authors: Ruchi S. Gupta, M.D., M.P.H., Northwestern University Feinberg School of Medicine and the Ann & Robert H. Lurie Children’s Hospital, Chicago, and coauthors
Visual Abstract

To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamanetworkopen.2018.5630)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Below is a visual abstract for the December 5 JAMA Surgery study, “Association of Prevalence of Benign Pathologic Findings After Partial Nephrectomy With Preoperative Imaging Patterns in the United States From 2007 to 2014,” by Jae Heon Kim, M.D., Ph.D., Soonchunhyang University Seoul Hospital, Seoul, South Korea and coauthors.
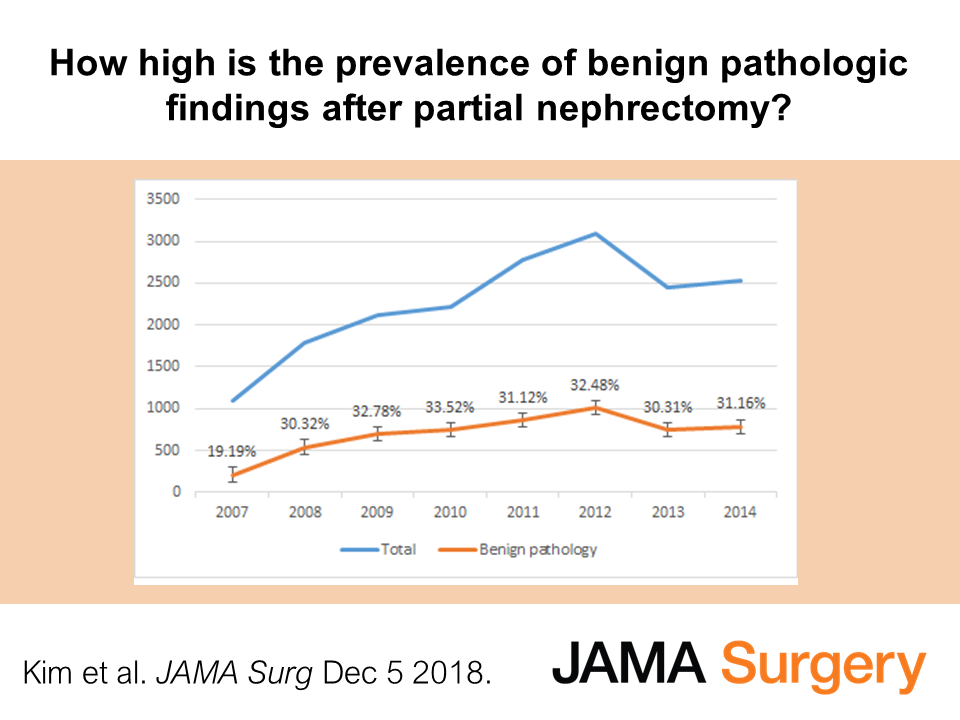
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, DECEMBER 5, 2018
Media Advisory: To contact corresponding author Ole Köhler-Forsberg, M.D., email karkoe@rm.dk. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time: https://jamanetwork.com/journals/jamapsychiatry/fullarticle/10.1001/jamapsychiatry.2018.3428
Bottom Line: This study used Danish nationwide registries to investigate an association between infections treated since birth and subsequent risk of treated childhood and adolescent mental disorders. Among nearly 1.1 million people born in Denmark between 1995 and 2012, about 42,000 (3.9 percent) were hospitalized for any mental disorder and nearly 57,000 (5.2 percent) redeemed a prescription for psychotropic medication. Infections requiring hospitalization were associated with subsequent increased risk of hospitalization for any mental disorder and increased risk of psychotropic medication use. Infections treated with medication, especially antibiotics, were associated with increased risk. Risks differed among mental disorders. Schizophrenia spectrum disorders, obsessive-compulsive disorder, personality and behavior disorders, mental retardation, autism spectrum disorder, attention-deficit/hyperactivity disorder, oppositional defiant disorder and conduct disorder, and tic disorders were associated with the highest risks after infections. This is an observational study and other factors might explain the results including the consequences of infections on the developing brain and other influences such as genetics and disturbances of the gut biome.
Authors: Ole Köhler-Forsberg, M.D., of Aarhus University Hospital, Denmark, and coauthors
Related Material: The editorial, “Harbingers of Mental Disease – Infections Associated With an Increased Risk for Neuropsychiatric Illness in Children,” by Viviane Labrie, Ph.D., and Lena Brundin, M.D., Ph.D., of the Van Andel Research Institute, Grand Rapids, Michigan, also is available on the For The Media website.
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/ jamapsychiatry.2018.3428)
Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, DECEMBER 5, 2018
Media advisory: To contact corresponding author Edward G. Soltesz, M.D., M.P.H., email Andrea Pacetti at PACETTA@ccf.org. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jamasurgery/fullarticle/10.1001/jamasurg.2018.4608
Bottom Line: Persistent use of opioids by patients is a public health concern in the United States but not much is known about the effect of that use on patients undergoing cardiac surgery. This observational study included 5.7 million patients who underwent cardiac surgery and it compared outcomes among those with persistent opioid use or dependence and those patients without. Researchers report no significant difference in the rate of death between the two groups of patients, although patients with persistent opioid use or dependence had a higher number of complications overall, longer length of hospital stay and higher costs. Limitations of the study include the possibility that opioid overuse disorders were underreported and that the definitions of opioid dependence or persistent opioid use weren’t consistent between hospitals.
Visual Abstract
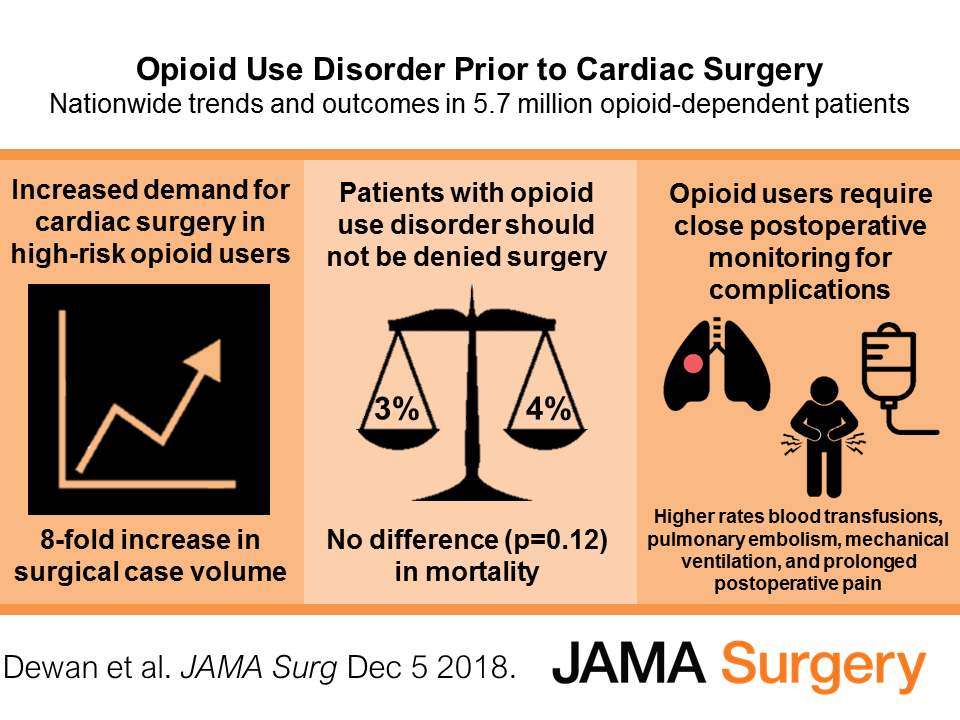
Authors: Edward G. Soltesz, M.D., M.P.H., Cleveland Clinic Foundation, Cleveland, Ohio, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamasurg.2018.4608)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, DECEMBER 3, 2018
Media advisory: To contact corresponding author Wei Bao, M.D., Ph.D., email Tom Snee at Tom-snee@uiowa.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jamapediatrics/fullarticle/10.1001/jamapediatrics.2018.4208
Bottom Line: National survey data for 43,000 U.S. children suggests an estimated 2.8 percent have ever been diagnosed with autism spectrum disorder (ASD) and 2.5 percent currently have ASD. Among 1,115 children with current ASD, almost 30 percent aren’t treated with behavioral therapies or medication. ASD is a neurodevelopmental disorder marked by social impairments, communication difficulties, repetitive behaviors and restricted interests. Symptoms of ASD are often treated with behavioral therapies and medications. Among children with ASD who were treated, almost 64 percent received behavioral treatment and 27 percent received medication. In the study, which used data from the 2016 National Survey of Children’s Health, the frequency of ASD among children varied by state. The study has limitations, including that physician diagnoses of ASD were self-reported by parents. Understanding why some children with ASD don’t receive treatment is important.
Authors: Wei Bao, M.D., Ph.D., of the University of Iowa, Iowa City, and coauthors
To Learn More: All the articles are available on the For The Media website.
(doi:10.1001/jamapediatrics.2018.4208)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, DECEMBER 3, 2018
Media advisory: To contact study author Alan R. Schroeder, M.D., email Erin Digitale at digitale@stanford.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Links will be live at the embargo time https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/10.1001/jamainternmed.2018.5419
Bottom Line: An analysis of claims data for privately insured adolescents and young adults suggests initial exposure to opioids prescribed by dental providers may be associated with increased risk of subsequent opioid use and abuse. Dentists are a leading source of opioid prescriptions for children and adolescents. This observational study examined outpatient opioid prescriptions for patients 16 to 25 in 2015 because that’s the common age when third molars show up and are extracted. Included in the study were 14,888 people in an opioid-exposed group because they had filled an opioid prescription from a dental provider and 29,776 in a control group not exposed to opioids. The study relied on diagnosis codes so some misclassification may have happened and the results may not be generalized to other insured patients. The study concludes that more scrutiny of third-molar extractions and opioid prescriptions associated with postoperative care is needed.
Authors: Alan R. Schroeder, M.D., of Stanford University School of Medicine, Stanford, California, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamainternmed.2018.5419)
Editor’s Note: The article contains funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or emailmediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, DECEMBER 3, 2018
Media advisory: To contact study author Kasia J. Lipska, M.D., M.H.S., email Ziba Kashef at ziba.kashef@yale.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Links will be live at the embargo time https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/10.1001/jamainternmed.2018.5008
Bottom Line: In a small survey of patients at an urban diabetes center, 1 in 4 reported skimping on their prescribed insulin because of cost and this was associated with poor glycemic control. Insulin is lifesaving for people with diabetes and is listed as an essential medicine by the World Health Organization, which means that it should be available at a price individuals and the community can afford. Insulin prices have increased substantially in the past decade in the United States and so have out-of-pocket prescription costs. Of the 199 patients with type 1 or type 2 diabetes who were prescribed insulin and who completed the survey, 51 (25.5 percent) reported cost-related underuse of insulin. Underuse included using less than prescribed, trying to stretch out insulin, smaller doses, stopping insulin, not filling a prescription or not starting prescribed insulin. More than a third of the patients with cost-related underuse didn’t discuss the matter with their doctor. The single-center study may be limited in its broader ability to be generalized but researchers conclude the results highlight an urgent need to address the affordability of insulin.
Authors: Kasia J. Lipska, M.D., M.H.S., of the Yale School of Medicine, New Haven, Connecticut, and coauthors
Related Material: The invited commentary, “When High Prices Mean Needless Death,” by Elisabeth Rosenthal, M.D. , of Kaiser Health News, Washington, D.C., also is available on the For The Media website.
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamainternmed.2018.5008)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or emailmediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, DECEMBER 4, 2018
Media advisory: To contact corresponding author Dowin H. Boatright, M.D., M.B.A., M.H.S., email Ziba Kashef at ziba.kashef@yale.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time: https://jamanetwork.com/journals/jama/fullarticle/10.1001/jama.2018.13705
Bottom Line: This observational study looked at changes in student makeup by sex, race and ethnicity at U.S. medical schools after an accrediting organization introduced diversity standards in 2009. An analysis of data from 120 medical schools suggests implementation of the diversity standards were associated with increasing percentages of female and black students. The study cannot demonstrate causality and other unaccounted factors could help explain the findings. Researchers noted the results are promising but that disparities persist in the diversity of the physician workforce.
Authors: Dowin H. Boatright, M.D., M.B.A., M.H.S., Yale School of Medicine, New Haven, Connecticut, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jama.2018.13705)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, DECEMBER 4, 2018
Media advisory: To contact corresponding author Anupam B. Jena, M.D., Ph.D., email Ekaterina Pesheva at Ekaterina_Pesheva@hms.harvard.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time: https://jamanetwork.com/journals/jama/fullarticle/10.1001/jama.2018.14270
Bottom Line: Health care professionals not born in the United States, including those who are noncitizens, made up a significant proportion of the health care workforce in 2016. An analysis of U.S. Census Bureau data for 164,000 health care professionals found 16.6 percent weren’t born in the United States and 4.6 percent were noncitizens. Non-U.S.-born health care professionals were a substantial proportions of several health care professions, including physicians (29 percent), dentists (24 percent), pharmacists (20 percent), registered nurses (16 percent) and nursing, psychiatric, and home health aides (23 percent). The majority of health care professionals not born in the United States were born in Asia (6.4 percent) or Mexico and Central America or the Caribbean (4.7 percent). The studied relied on survey-reported occupation and there was the possibility of underreporting of noncitizenship.
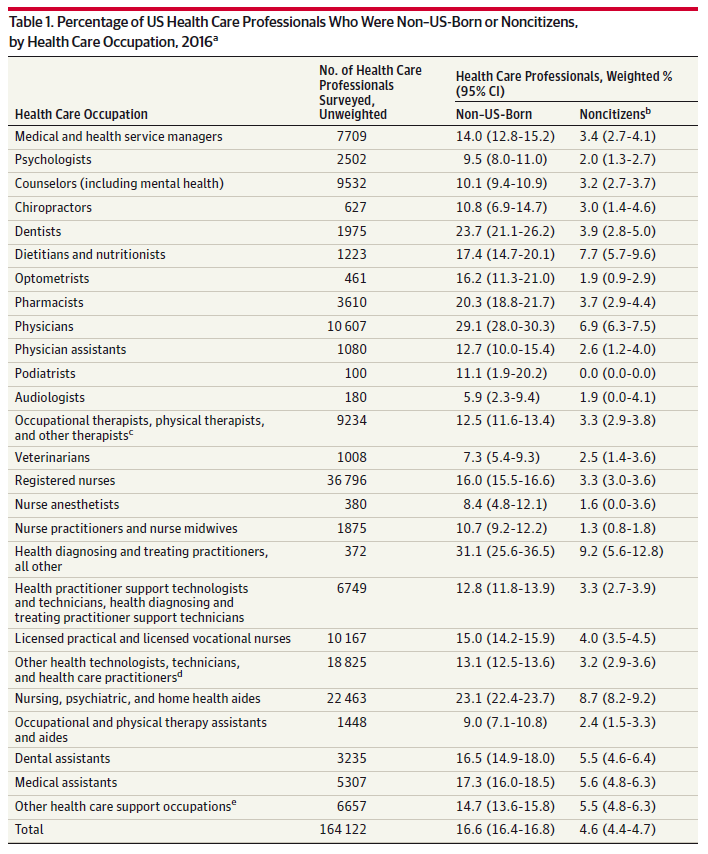
Authors: Anupam B. Jena, M.D., Ph.D., Harvard Medical School, Boston, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jama.2018.14270)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, NOVEMBER 30, 2018
Media advisory: To contact corresponding author Matthew J. Elrick. M.D., Ph.D., email Vanessa McMains at vmcmain1@jhmi.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jamapediatrics/fullarticle/10.1001/jamapediatrics.2018.4890
Bottom Line: Acute flaccid myelitis (AFM) is a poorly understood polio-like illness mostly of children characterized by weakness of muscles and limbs and the presence of a spinal cord lesion. An increase in cases was first suspected in 2012 and some epidemiologic evidence suggests viruses may be associated with AFM outbreaks in the United States in the late summer and fall of 2014, 2016 and 2018. Much still needs to be learned about the cause, progression, biomarkers, prognosis and treatment of this rare condition.
JAMA Pediatrics published three new articles on AFM because of their clinical and public health importance:

To Learn More: All the articles are available on the For The Media website.
(doi:10.1001/jamapediatrics.2018.4890)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), THURSDAY, NOVEMBER 29, 2018
Media advisory: To contact corresponding author Akira Ohtsuru, M.D., Ph.D., email ohtsuru@fmu.ac.jp. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jamaotolaryngology/fullarticle/10.1001/jamaoto.2018.3121
Bottom Line: The accident at Japan’s Fukushima Daiichi nuclear power station in 2011 raised grave concerns about radioactive material released into the environment, including concerns over radiation-induced thyroid cancer. Ultrasound screenings for thyroid cancer were subsequently conducted in the Fukushima Health Management Survey. This observational study group includes about 324,000 people 18 or younger at the time of the accident and it reports on two rounds of ultrasound screening during the first five years after the accident. Thyroid cancer or suspected cancer was identified in 187 individuals within five years (116 people in the first round among nearly 300,000 people screened and 71 in the second round among 271,000 screened). The overwhelmingly common diagnosis in surgical cases was papillary thyroid cancer (149 of 152 or 98 percent).
Authors: Akira Ohtsuru, M.D., Ph.D., Fukushima Medical University, Fukushima, Japan, and coauthors
Related material: The commentary, “Why the Data From the Fukushima Health Management Survey After the Daiichi Nuclear Power Station Accident Are Important,” by Andrew J. Bauer, M.D., University of Pennsylvania, Philadelphia, and Louise Davies, M.D., M.S., Department of Veterans Affairs Medical Center, White River Junction, Vermont, is available on the For The Media website.
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamaoto.2018.3121)
Editor’s Note: The article includes conflict of interest disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, NOVEMBER 30, 2018
Media advisory: To contact corresponding study author Renee Y. Hsia, M.D., M.Sc., email Elizabeth Fernandez at elizabeth.fernandez@ucsf.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story?: Links will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2018.5202
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: Patients from the poorest neighborhoods who had cardiac arrest had longer total ambulance times than those from the wealthiest neighborhoods.
Why The Research Is Interesting: Emergency medical services (EMS) provide critical care before patients reach the hospital and differences in ambulance times may contribute to disparities in patient outcomes.
What and When: National data from 46 states on 63,600 patients who had cardiac arrest and didn’t die on scene and were transported to a hospital
What (Study Measures and Outcomes): Four time measures were examined (response time, on-scene time, transport time and total EMS time) and compared with EMS response time benchmarks for responding to cardiac arrest calls.
How (Study Design): This was an observational study. Researchers weren’t intervening for purposes of the study and cannot control all the natural differences that could explain the study findings.
Authors: Renee Y. Hsia, M.D., M.Sc., University of California, San Francisco, and coauthors
Results:

Study Limitations: The registry analyzed for this study wasn’t of individual patients so multiple reports associated with the same patient exist; other explanations beyond the variables assessed in this study may have contributed to time disparities; and the findings may not be generalized to other types of time-sensitive EMS calls.
Study Conclusions:

Related Material: The invited commentary, “Income and Ambulance Response Time Inequality—No Simple Explanation, No Simple Fix,” by Andrew I. Friedson, Ph.D., University of Colorado Denver, also is available on the For The Media website.
Visual Abstract: This visual abstract also is available on the For The Media website.
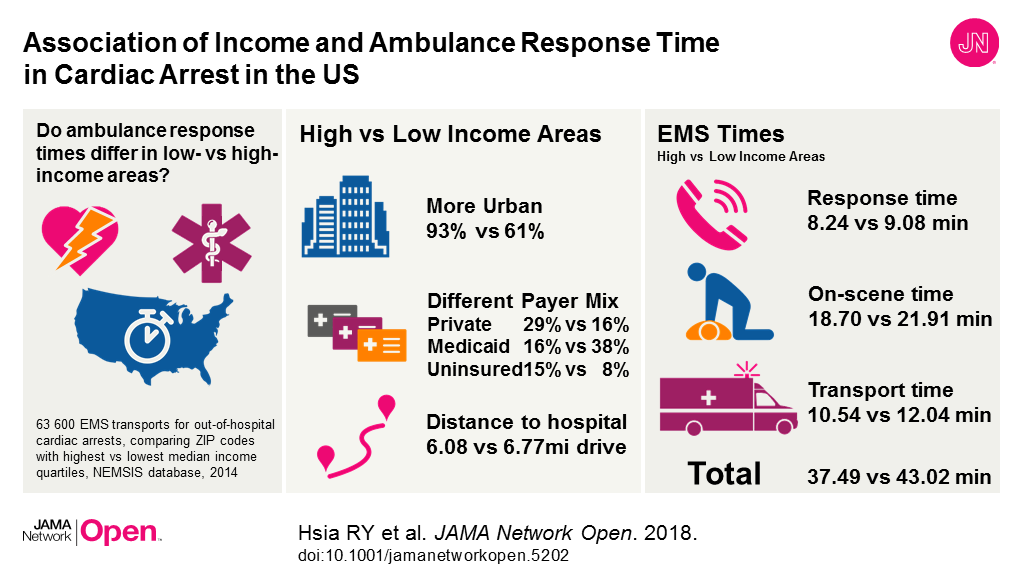
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamanetworkopen.2018.4945)
Editor’s Note: The article contains funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.