JAMA Internal Medicine
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, SEPTEMBER 10, 2018
Media advisory: To contact corresponding author Anna L. Goldman, M.D., M.P.H., email David Cecere at dcecere@challiance.org and to contact corresponding author David M. Silvestri, M.D., M.B.A., email Anne Doerr at anne.doerr@yale.edu. The full articles are available on the For The Media website.
Want to embed a link to this study in your story? Links will be live at the embargo time: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/10.1001/jamainternmed.2018.4194 (Goldman study) and https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/10.1001/jamainternmed.2018.4196 (Silvestri study)
Bottom Line: Two research letters and an invited commentary examine work requirements for Medicaid recipients, a move favored by some states that have federal waivers or have applied for them to impose work rules.
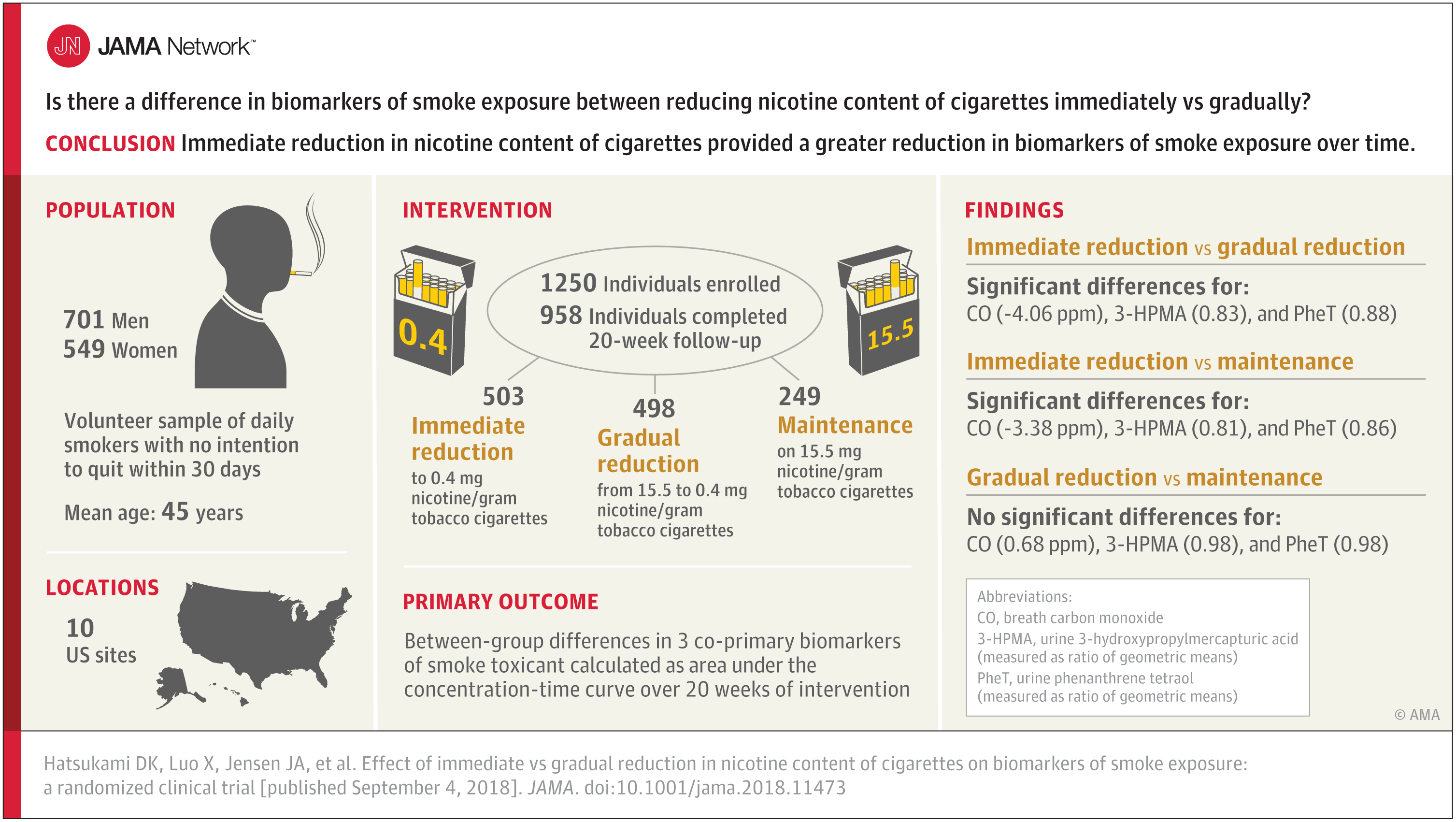
One research letter estimated the number of Medicaid recipients at risk of losing coverage if work requirements were applied nationally and in states with approved or pending waivers to impose those rules. The article also calculated Medicaid spending for those at risk of losing Medicaid coverage. Authors: Anna M. Goldman, M.D., M.P.H., of the Cambridge Health Alliance and the Harvard T.H. Chan School of Public Health, Boston, Massachusetts, and coauthors
A second research letter provides state-level population estimates of people who are subject to but not meeting proposed Medicaid work requirements. Authors: David M. Silvestri, M.D., M.B.A., of the Yale School of Medicine, New Haven, Connecticut, and coauthors
Related Material: The invited commentary, “Medicaid Work Requirements – English Poor Law Revisited,” by Dave A. Chokshi, M.D., M.Sc., and Mitchell H. Katz, M.D., of the NYC Health + Hospitals, also is available on the For The Media website. Dr. Katz is a JAMA Internal Medicine deputy editor.
To Learn More: The full articles are available on the For The Media website.
(doi:10.1001/jamainternmed.2018.4194 and doi:10.1001/jamainternmed.2018.4196)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or emailmediarelations@jamanetwork.org.