Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2019.6553?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=062819
Here’s a link to provide your readers free access to the full-text article
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2019.6419?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=062819
Here’s a link to provide your readers free access to the full-text article
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2019.6587?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=062819
Statewide Action in California Associated With Decrease in Kindergartners Entering School Without Up-To-Date Vaccines
JAMA
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, JULY 2, 2019
Media advisory: To contact corresponding author S. Cassandra Pingali, M.P.H., M.S., email Janet Christenbury at jmchris@emory.edu. The full study, editorial and podcast are linked to this news release.
Embed this link to provide your readers free access to the full-text article This link will be live at the embargo time: https://jamanetwork.com/journals/jama/fullarticle/2737172?guestAccessKey=779b1f3f-bf59-48a2-8882-233c98595720&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=070219
Bottom Line: Legislative and administrative actions by the state of California were associated with a decrease in the rate of kindergartners entering school without up-to-date vaccinations. This observational study focused on three statewide initiatives: a 2014 bill requiring parents to prove they had discussed the risks of not vaccinating their children with a health care provider before getting a personal belief exemption; a 2015 educational campaign by state and local health departments to educate school staff on conditional admission criteria that allow students more time to catch up on vaccinations; and a 2016 bill banning all personal belief exemptions for vaccinations. Researchers used school entry data to calculate rates of kindergartners attending California schools without up-to-date vaccines. The authors report the rate of kindergartners without up-to-date vaccinations decreased from 9.84% during 2013 (before the three statewide interventions) to 4.87% in 2017 (after the interventions). Limitations of the study include a limited time period for examining each intervention and students with varying vaccination status not specific to particular vaccines.
Authors: S. Cassandra Pingali, M.P.H., M.S., Emory University. Atlanta, and coauthors
(doi:10.1001/jama.2019.7924)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
What Is Association of Radioactive Iodine Treatment for Overactive Thyroid With Risk of Cancer Death?
JAMA Internal Medicine
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JULY 1, 2019
Media advisory: To contact corresponding author Cari M. Kitahara, Ph.D., email NCIPressOfficers@mail.nih.gov. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article This link will be live at the embargo time: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2737319?guestAccessKey=6f287130-e597-4e48-b8a6-295bd94dc1fb&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=070119
Bottom Line: Radioactive iodine has been used since the 1940s to treat hyperthyroidism, an overactive thyroid. This study is an extension of one that has followed patients in the United States and the United Kingdom treated for hyperthyroidism for nearly 70 years. Researchers sought to determine the association of doses of radioactive iodine absorbed by organs or tissue with overall and site-specific cancer death. This analysis included 18,805 patients treated with radioactive iodine and with no history of cancer at the time of treatment. Researchers report a modest association between greater organ-absorbed doses of radioactive iodine and risk of death from solid cancer (a mass), including breast cancer. The study has limitations, including uncertainties in the organ dose estimates and a limited ability to detect significant associations for some outcomes because of a small number of cancer deaths and relatively small doses of radioactive iodine to organs other than the thyroid. More studies are needed to compare the risks and advantages of all major treatment options for patients with hyperthyroidism.
Authors: Cari M. Kitahara, Ph.D., National Institutes of Health, Bethesda, Maryland, and coauthors
(doi:10.1001/jamainternmed.2019.0981)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Study Examines Changes in Health Equity in U.S. Over 25 Years
JAMA Network Open
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, JUNE 28, 2019
Media advisory: To contact corresponding author Frederick J. Zimmerman, Ph.D., email Carla Denly at cdenly@support.ucla.edu. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2019.6386?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=062819
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: A survey study based on 25 years of data from more than 5.4 million people in the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System suggests more work is needed on health equity in the United States. The study assessed health equity for healthy days and self-reported health, using a novel measure of health equity as well as the disparities gap between black and white individuals, income disparities and health justice (a measure of how health outcomes correlate with income, race/ethnicity and sex). National estimates of change from 1993 to 2017 suggest downward movement in average health; improvement in the disparities gap between black and white individuals; a decline in other measures of health equity and health justice; and worsening income disparities. The study has limitations in its data. Study authors suggest more or different approaches are needed to improve health equity.
Authors: Frederick J. Zimmerman, Ph.D., and Nathaniel W. Anderson, B.A., of the University of California, Los Angeles
(doi:10.1001/jamanetworkopen.2019.5529)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Birth, Child Outcomes Associated With Moms Using Opioids During Pregnancy
JAMA Network Open
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, JUNE 28, 2019
Media advisory: To contact corresponding author Romuladus E. Azuine, Dr.P.H., M.P.H., R.N., email the Health Resources and Services Administration Press Office at press@hrsa.gov. The full study and commentary are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2019.6405?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=062819
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: In utero exposure to opioids was associated with higher risks for short- and long-term adverse outcomes including preterm birth and neurodevelopmental and physical health disorders in children. This observational study analyzed clinical and epidemiological data for a group of 8,509 mother-child pairs collected at birth starting in 1998, and 3,153 children who continued to be followed after birth up to age 21 years old. Of the 8,509 children, 454 (5.3%) had in utero opioid exposure, which was defined as maternal self-reported opioid use or a clinical diagnosis of neonatal abstinence syndrome for a child. The study reports that in utero exposure to opioids was associated with a higher likelihood of being small for gestational age and preterm birth. In utero exposure to opioids also was associated with postnatal neurodevelopmental and physical disorders, including a higher likelihood of conduct disorder or emotional disturbance diagnoses, as well as lack of normal physiological development in children before age 6 years old, and later on, a higher likelihood of attention-deficit/hyperactivity disorder. Study limitations to consider include that mothers may have used other substances, such as alcohol, cigarettes, marijuana and stimulants, which could have influenced the outcomes.
Authors: Romuladus E. Azuine, Dr.P.H., M.P.H., R.N., U.S. Department of Health and Human Services, Rockville, Maryland, and coauthors
(doi:10.1001/jamanetworkopen.2019.6405)
Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Is Use of Social Media, Photo Editing Apps Associated With Acceptance of Cosmetic Surgery?
JAMA Facial Plastic Surgery
EMBARGOED FOR RELEASE: 11 A.M. (ET), THURSDAY, JUNE 27, 2019
Media advisory: To contact corresponding author Lisa E. Ishii, M.D., M.H.S., email email Michael Newman at mnewma25@jhmi.edu. The full study and commentary are linked to this news release.
Embed this link to provide your readers free access to the full-text article This link will be live at the embargo time: https://jamanetwork.com/journals/jamafacialplasticsurgery/fullarticle/2736534?guestAccessKey=c6512ff2-7556-4d9c-8fdc-91de30f634cc&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=062719
Bottom Line: An online survey study suggests how people feel about cosmetic surgery may be associated with what social media and photo editing apps they use. Most of the 252 survey participants were white and women, with an average age of almost 25, and had not previously undergone any cosmetic surgeries. Self-esteem and acceptance of cosmetic surgery attitudes were measured. YouTube and WhatsApp social media users had lower self-esteem scores than nonusers, as did photo editing platforms users of VSCO and Photoshop. Users of Tinder, Snapchat and Snapchat filters had higher overall acceptance of cosmetic surgery scores. These findings could help to inform discussions between patients and physicians regarding expectations and outcomes of cosmetic surgery. However, the results aren’t representative of most patients seeking cosmetic surgery because of the young age of survey participants.
Authors: Lisa E. Ishii, M.D., M.H.S., Johns Hopkins University School of Medicine, Baltimore, and coauthors
(doi:10.1001/jamafacial.2019.0328)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Evaluation of USPSTF Lung Cancer Screening Guidelines for African American Smokers
JAMA Oncology
EMBARGOED FOR RELEASE: 11 A.M. (ET), THURSDAY, JUNE 27, 2019
Media advisory: To contact corresponding author Melinda C. Aldrich, Ph.D., email Craig Boerner at craig.boerner@vumc.org. The full study and a podcast are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamaoncology/fullarticle/2737091?guestAccessKey=e82549cb-b4d5-4460-827f-1b07b47eab76&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=062719
Bottom Line: An observational study suggests the U.S. Preventive Services Task Force (USPSTF) lung cancer screening guidelines may be too conservative for African American smokers and that some eligibility criteria changes could result in more screenings of African American smokers at high risk for lung cancer. The study looked at new lung cancer cases in a predominantly low-income and African American population group to assess their eligibility for lung cancer screening using the USPSTF criteria. The USPSTF routinely makes recommendations about the effectiveness of preventive care services. This study included 48,364 adults who ever smoked (67% were African American) and 1,269 new lung cancers were identified. Among the smokers, 17% of African American smokers were eligible for USPSTF screening compared with 31% of white smokers. The lower percentage of lung cancer cases eligible for screening among African American smokers was largely associated with fewer smoking pack-years (a measure of smoking) among African American smokers compared with white smokers. African Americans tend to smoke fewer cigarettes per day and tend to have a lower overall smoking pack-year history compared with white smokers. These study results suggest that lowering the smoking pack-year eligibility requirement from 30 to 20 pack years for African American smokers could increase the number of African American smokers eligible for screening. In addition, reducing the minimum age criterion for screening to 50 for African American smokers could further increase eligibility. The average age of a lung cancer diagnosis tends to be earlier for African American smokers compared with white smokers. This study has limitations to consider, including that smoking was self-reported and authors didn’t have information about actual lung cancer screening use.
Authors: Melinda C. Aldrich, Ph.D., of the Vanderbilt University Medical Center, Nashville, Tennessee, and coauthors
(doi:10.1001/jamaoncol.2019.1402)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Test for Alzheimer Disease–Related β-Amyloid Status
JAMA Neurology
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JUNE 24, 2019
Media Advisory: The fully study and editorial are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamaneurology/fullarticle/2736342?guestAccessKey=11bfad02-0f3b-4024-831d-976ff34c3fea&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=062419
Authors: Sebastian Palmqvist, M.D., Ph.D., and Oskar Hansson, M.D., of Skåne University Hospital in Malmö, Sweden, are the corresponding authors.
(doi:10.1001/jamaneurol.2019.1632)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Does Likelihood of Survival Differ Between Patients With Single vs. Multiple Primary Melanomas?
JAMA Dermatology
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, JUNE 26, 2019
Media advisory: To contact corresponding author Mary-Ann El Sharouni, M.D., email m.a.elsharouni-2@umcutrecht.nl. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article This link will be live at the embargo time: https://jamanetwork.com/journals/jamadermatology/fullarticle/2736968?guestAccessKey=285c6cb0-c551-4af0-9caf-ca3c2ab26bf4&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=062619
Bottom Line: Patients with multiple primary melanomas had a higher likelihood of dying than those with a single primary melanoma in a study that used data from registries in the Netherlands. This observational study included nearly 57,000 patients (54,645 with a single primary melanoma and 2,284 with multiple primary melanomas). The study has limitations to consider, including a lack of information about family history and another possible limitation that researchers chose not to include melanoma in situ (when the disease is in its earliest form) because they analyzed features not related to it. The findings suggest the possibility that more strict follow-up may be warranted for patients with multiple primary melanomas.
Authors: Mary-Ann El Sharouni, M.D., University Medical Center Utrecht, the Netherlands, and coauthors
(doi:10.1001/jamadermatol.2019.1134)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Underage Sales Violations in Tobacco Stores, Vape Shops
JAMA Pediatrics
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JUNE 24, 2019
Media advisory: The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamapediatrics/fullarticle/2735684?guestAccessKey=70d28f80-22da-4324-8857-476323e86d64&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=062419
Authors: April Roeseler, B.S.M., M.S.P.H., of the California Department of Public Health in Sacramento, is the corresponding author.
(doi:10.1001/jamapediatrics.2019.1571)
Editor’s Note: The article contains conflict of interest disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Anticholinergic Drug Exposure and Dementia Risk
JAMA Internal Medicine
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JUNE 24, 2019
Media advisory: The full study and commentary are linked to this news release.
Embed this link to provide your readers free access to the full-text article This link will be live at the embargo time: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2736353?guestAccessKey=2eaed393-41eb-4a06-b3f6-6ee3855f0bb1&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=062419
Authors: Carol A.C. Coupland, Ph.D., of the University of Nottingham in England, is the corresponding author.
(doi:10.1001/jamainternmed.2018.0677)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Opioid Overdose More Likely if Family Member Has Opioid Prescription
JAMA Internal Medicine
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JUNE 24, 2019
Media advisory: To contact corresponding author Joshua J. Gagne, Pharm.D., Sc.D., email Elaine St. Peter at estpeter@bwh.harvard.edu. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article This link will be live at the embargo time: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2736355?guestAccessKey=d6ac0318-1516-43ea-88aa-31e86fa163c9&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=062419
Bottom Line: Having a family member who was previously dispensed prescription opioids was associated with higher odds of overdose for individuals who themselves didn’t have an opioid prescription in this analysis of insurance company data. The study included 2,303 people with the earliest date of an opioid overdose in a family and 9,212 others in the insurance database for comparison. Researchers report the increased risk of opioid overdose for an individual if a family member was prescribed an opioid applied to all age groups and increased with greater quantities of opioids prescribed. Potential interventions include encouraging patients to properly dispose of or secure prescription opioids in their homes, as well as improving patient and public education. Limitations of the study include that the time period analyzed (through 2015) may not reflect current patterns of opioid prescribing or overdoses.
Authors: Joshua J. Gagne, Pharm.D., Sc.D., Brigham and Women’s Hospital, Harvard Medical School, Boston, and coauthors
(doi:10.1001/jamainternmed.2019.1064)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Is U.S. Immigration Policy Environment Associated With Mental Health Outcomes for U.S.-Born Teens of Immigrant Parents?
JAMA Pediatrics
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JUNE 24, 2019
Media advisory: To contact corresponding author Brenda Eskenazi, Ph.D., email Kara Manke at kjmanke@berkeley.edu. The full study and editorial are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamapediatrics/fullarticle/2735685?guestAccessKey=1cdd4743-8c9a-409b-b62b-575c55f23243&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=062419
Bottom Line: The current immigration policy environment in America appears to be associated with reported adverse mental health outcomes among U.S.-born children of Latinx immigrants. Data were used from a group of 397 U.S.-born adolescents with at least one immigrant parent from a long-term study of Mexican farmworker families in the Salinas Valley of California. Researchers examined associations between adolescent self-reported concerns about immigration policy collected at age 16 on an assessment tool and changes in their mental and physical health before (when they were 14) and in the first year after the 2016 election (when they were 16). Nearly half of the Latinx adolescents were worried at least sometimes about the personal consequences of U.S. immigration policy, family separation because of deportation, and being reported to the immigration office. High (versus low or moderate) scores on the assessment about concerns over immigration policy were associated with higher anxiety and worse sleep scores. A limitation of the study to consider is that researchers didn’t know the immigration status of parents.
Author: Brenda Eskenazi, Ph.D., of the University of California, Berkeley, and coauthors
(doi:10.1001/jamapediatrics.2019.1475)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Food Insecurity Associated With Migraine in Young U.S. Adults
JAMA Neurology
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JUNE 24, 2019
Media Advisory: To contact corresponding author Jason M. Nagata, M.D., M.Sc., email Suzanne Leigh at suzanne.leigh@ucsf.edu. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamaneurology/fullarticle/2736340?guestAccessKey=a8a03217-f038-4627-9e04-bdefc9788dd3&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=062419
Bottom Line: Food insecurity is when you worry that your food will run out before you have enough money to buy more. This study used nationally representative data to examine the association between food insecurity and migraine in young U.S. adults because the economic and education transition of young adulthood may increase risk for food insecurity. The study included almost 14,800 young people (ages 24 to 32). Overall, 11% of young adults were food insecure, and migraine was more common among young adults who were food insecure. Food insecurity may lead to some migraine triggers, including missed meals, stress, depression and poor sleep. And, migraine may contribute to food insecurity by leading to poor attendance and productivity at work, resulting in lost employment. Researchers suggest clinicians screen for food insecurity among people with migraine.
Authors: James M. Nagata, M.D., M.Sc., of the University of California, San Francisco, and coauthors
(doi:10.1001/jamaneurol.2019.1663)
Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Study Looks at Opioid Use After Knee Surgery
JAMA
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, JUNE 25, 2019
Media advisory: To contact corresponding author John Xerogeanes, M.D., email Elaine Justice at EJUSTIC@emory.edu. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jama/fullarticle/2736546?guestAccessKey=8b735847-2d8e-469a-b84a-8f53c33b725f&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=062519
Bottom Line: A small study looked at whether reducing the number of opioid tablets prescribed after knee surgery would reduce postoperative use and if preoperative opioid-use education would reduce it even more. The study included 264 patients who underwent anterior cruciate ligament (ACL) surgery at a single academic ambulatory surgery center. They were divided into three groups: 109 were prescribed 50 opioid tablets after surgery; 78 patients were prescribed 30 tablets and before surgery received education on appropriate opioid use and alternative pain control strategies; and 77 patients received 30 tablets and no education. Patients were surveyed about their opioid use three weeks after surgery. Researchers report patients who received 50 tablets consumed more tablets (an average of 25) and for more days (nearly 6) than those given 30 tablets and no education who consumed less (an average of about 16) and for fewer days (about 4½). Patients who received 30 tablets and preoperative education used fewer tablets (average of about 12) and for fewer days (about 3½) than patients who received 30 tablets but no opioid use education. A limitation of the study to consider is that was conducted at just one surgery center and the patient group was young.
Authors: John Xerogeanes, M.D., Emory University School of Medicine, Atlanta, and coauthors
(doi:10.1001/jama.2019.6125)
Editor’s Note: The article includes conflict of interest disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Estimate of Nutrient Intake Among Pregnant Women in U.S.
JAMA Network Open
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, JUNE 21, 2019
Media advisory: The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2019.5967?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=062119
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
What The Study Did: This observational study used nationally representative survey data to estimate nutrient intake from food and dietary supplements for about 1,000 pregnant women in the U.S. to see how it compared to recommendations from the National Academies of Science, Engineering and Medicine Dietary Reference Intakes.
Authors: Regan L. Bailey, Ph.D., M.P.H., R.D., of Purdue University in Lafayette, Indiana, is the corresponding author
(doi:10.1001/jamanetworkopen.2019.6160)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Analysis of Pharmaceutical Industry Payments to UK Health Care Organizations in 2015
JAMA Network Open
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, JUNE 21, 2019
Media advisory: The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2019.6253?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=062119
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Authors: Piotr Ozieranski, Ph.D., of University of Bath in the United Kingdom, is the corresponding author.
(doi:10.1001/jamanetworkopen.2019.6253)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Retinopathy Among Patients with Yellow Fever in Brazil
JAMA Ophthalmology
EMBARGOED FOR RELEASE: 11 A.M. (ET), THURSDAY, JUNE 20, 2019
Media advisory: The full study and commentary are linked to this news release.
Embed this link to provide your readers free access to the full-text article This link will be live at the embargo time: https://jamanetwork.com/journals/jamaophthalmology/fullarticle/2735632?guestAccessKey=c83f2de1-4c73-4086-b5d0-abb8f7443315&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=062019
Authors: Daniel V. Vasconcelos-Santos, M.D., Ph.D., of the Universidade Federal de Minas Gerais in Belo Horizonte, Brazil, is the corresponding author.
(doi:10.1001/jamaophthalmol.2019.1956)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Eye Exams Common Among U.S. Adults But Some Disparities Persist
JAMA Ophthalmology
EMBARGOED FOR RELEASE: 11 A.M. (ET), THURSDAY, JUNE 20, 2019
Media advisory: To contact corresponding author Joshua R. Ehrlich, M.D., M.P.H., email Shantell Kirkendoll at smkirk@med.umich.edu. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamaophthalmology/fullarticle/2735627?guestAccessKey=a405ba2b-4bab-4d80-aa40-40bfffa64c57&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=062019
Bottom Line: A substantial proportion of U.S. adults reported recently having an eye exam in this online survey study that included 2,013 adults ages 50 to 80. About 82% of those surveyed reported an eye exam in the past two years. Reasons for not getting an exam included not having eye problems, cost or lack of insurance. A limitation of the study is that adults with vision impairment may have been less likely to participate because the survey was administered online.
Authors: Joshua R. Ehrlich, M.D., M.P.H., University of Michigan, Ann Arbor, and coauthors
(doi:10.1001/jamaophthalmol.2019.1927)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Rate of Memory Change Before and After Cancer Diagnosis
JAMA Network Open
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, JUNE 21, 2019
Media advisory: The full study and commentary are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2019.6160?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=062119
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
What The Study Did: This observational study used a composite memory score to compare the rate of memory change among people with cancer before and after diagnosis with the rate of memory change among people who remained cancer free during an average follow-up of almost 12 years.
Authors: M. Maria Glymour, Sc.D., M.S., of the University of California, San Francisco, is the corresponding author
(doi:10.1001/jamanetworkopen.2019.6160)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
3 Articles Focus on Marijuana Use During Pregnancy, Outcomes for Mother, Infant
JAMA
EMBARGOED FOR RELEASE: 3:45 P.M. (ET), TUESDAY, JUNE 18, 2019
Media advisory: The full study, research letter and editorial are linked to this news release. These are being published to coincide with a meeting presentation.
Embed these links to provide your readers free access to the full-text articles These links will be live at the embargo time:
Original Investigation: Association Between Self-reported Prenatal Cannabis Use and Maternal, Perinatal, and Neonatal Outcomes https://jamanetwork.com/journals/jama/fullarticle/2736583?guestAccessKey=488ef350-2589-4f25-b900-9b7ef51ae44d&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=061819
Research Letter: Self-reported Medical and Nonmedical Cannabis Use Among Pregnant Women in the United States https://jamanetwork.com/journals/jama/fullarticle/2736582?guestAccessKey=0fd8ac4c-b05c-4ae2-a209-5b7f31dcfea9&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=061819
(doi:10.1001/jama.2019.8734 and 10.1001/jama.2019.7982)
Editor’s Note: Please see the articles for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Barriers to Telehealth Programs and Dermatological Care for American Indian Communities
JAMA Dermatology
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, JUNE 19, 2019
Media advisory: The full study and editorial are linked to this news release.
Embed this link to provide your readers free access to the full-text article This link will be live at the embargo time: https://jamanetwork.com/journals/jamadermatology/fullarticle/2736373?guestAccessKey=86ea680e-e99d-4cf3-8f1e-e714cdde33cf&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=061919
Authors: Matthew Tobey, M.D., M.P.H., of Massachusetts General Hospital in Boston, is the corresponding author
(doi:10.1001/jamadermatol.2019.0872)
Editor’s Note: The article includes conflict of interest disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Certain Behaviors in Kindergarten Associated With Lower Adult Salary
JAMA Psychiatry
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, JUNE 19, 2019
Media advisory: To contact corresponding author Sylvana M. Cote, Ph.D., email Julie Gazaille at j.cordeau-gazaille@umontreal.ca. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article This link will be live at the embargo time: https://jamanetwork.com/journals/jamapsychiatry/fullarticle/2736346?guestAccessKey=645df423-f441-4424-a100-24008632a58a&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=061919
Bottom Line: Inattention among kindergarteners was associated with lower earnings as adults in this study based on behavioral ratings from kindergarten teachers for 2,850 children in Canada at ages 5 or 6 and government tax returns for those same children as adults at ages 33 to 35. Researchers report that after accounting for IQ and family background, kindergarten inattention was associated with lower earning for boys and girls later in life, while kindergarten ratings of aggression and opposition (disobeying, refusing to share, blaming others) were associated with lower earnings only for boys. The results suggest early monitoring and support for children demonstrating certain behaviors could have long-term benefits. A limitation of the study is that it shows only associations and causal inferences can’t be made.
Authors: Sylvana M. Cote, Ph.D., Universite de Montreal, and coauthors
(doi:10.1001/jamapsychiatry.2019.1326)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Reports of Unprofessional Behavior by Surgeons and Risk of Complications for Patients
JAMA Surgery
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, JUNE 19, 2019
Media advisory: To contact corresponding author William O. Cooper, M.D., M.P.H., email Craig Boerner at craig.boerner@vumc.org. The full study, commentary and podcast are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamasurgery/fullarticle/2736337?guestAccessKey=5bc0bbc7-9062-44e6-9c45-94eec1829251&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=061919
Bottom Line: This observational study looked at whether patients whose surgeons were more often reported by coworkers for unprofessional behavior were at greater risk of postoperative complications. The analysis included data from reports of unprofessional behavior by coworkers for 202 surgeons from two academic medical centers, as well as data on surgical and medical complications within 30 days of operation for 13,653 patients. Reported unprofessional behavior included concerns about poor or unsafe care, clear and respectful communication, and integrity. Among the patients, 1,583 (11.6%) experienced a complication. The study authors report patients whose surgeons had more reports by coworkers of unprofessional behavior were more likely to experience a complication. A limitation of the study to consider is that reporting of the observed behaviors may be subjective.
Author: William O. Cooper, M.D., M.P.H., Vanderbilt University School of Medicine, Nashville, and coauthors
(doi:10.1001/jamasurg.2019.1738)
Editor’s Note: The article contains funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Associations Between Birth Defect, Childhood Cancer Risk Examined
JAMA Oncology
EMBARGOED FOR RELEASE: 11 A.M. (ET), THURSDAY, JUNE 20, 2019
Media advisory: To contact corresponding author Philip J. Lupo, Ph.D., M.P.H., email Dana Benson at benson@bcm.edu. The full study and editorial are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamaoncology/fullarticle/2736368?guestAccessKey=ec26a29e-e3e0-445a-937c-b41d1c4df2eb&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=062019
Bottom Line: A study of more than 10 million births across four states suggests children with chromosomal anomalies and those with nonchromsomal birth defects were more likely to be diagnosed with cancer than those children without, however the overall absolute risk of cancer was small at less than 1%. This study used pooled statewide data on births, birth defects and cancer from Texas, Arkansas, Michigan and North Carolina for children born from 1992 through 2013 and followed up to when they were 18 years old for a cancer diagnosis. Overall, nearly 540,000 children were diagnosed with at least one birth defect. Among children with nonchromosomal birth defects, an increasing number of birth defects was associated with a corresponding increase in the risk of cancer before age 18, although the absolute risk of developing cancer remained small because childhood cancer is a rare outcome. The authors of this study also examined specific birth defect-childhood cancer associations. Limitations of this observational study to be considered include a lack of uniformity among states in birth defect surveillance.
Authors: Philip J. Lupo, Ph.D., M.P.H., of the Baylor College of Medicine, Houston, and coauthors
(doi:10.1001/jamaoncol.2019.1215)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Vitamin D Supplementation Not Associated With Reduced Cardiovascular Events
JAMA Cardiology
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, JUNE 19, 2019
Media advisory: To contact corresponding author Mahmoud Barbarawi, M.D., email Sarina Gleason at Sarina.Gleason@cabs.msu.edu. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article This link will be live at the embargo time: https://jamanetwork.com/journals/jamacardiology/fullarticle/2735646?guestAccessKey=d3f9efa6-cc55-45e0-a46f-e202dddd5ad8&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=061919
Bottom Line: This study, called a meta-analysis, combined the results of 21 randomized clinical trials with about 83,000 patients to look at whether vitamin D supplementation was associated with reduced risk of cardiovascular disease events such as heart attack or stroke. Some observational studies have suggested an association between low blood levels of vitamin D and an increased risk of cardiovascular disease events. This study reports that compared with placebo, vitamin D supplementation wasn’t associated with a reduction in major adverse cardiovascular events (heart attack, stroke or death from cardiovascular disease) or overall death. The results were similar between different doses of vitamin D and for men and women. A limitation of the study is that the definition of major adverse cardiovascular events varied between the clinical trials.
Authors: Mahmoud Barbarawi, M.D., Michigan State University, Flint, and coauthors
(doi:10.1001/jamacardio.2019.1870)
Editor’s Note: The article contains conflict of interest disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Association Between Transcatheter Aortic Valve Replacement and Early Postprocedural Stroke
JAMA
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, JUNE 18, 2019
Media advisory: The full study and editorial are linked to this news release.
Embed this link to provide your readers free access to the full-text article This link will be live at the embargo time: https://jamanetwork.com/journals/jama/fullarticle/2735820?guestAccessKey=4ac3deb7-cae3-49ae-be7f-b2f0a94b7aac&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=061819
Authors: Samir R. Kapadia, M.D., of the Cleveland Clinic, is the corresponding author
(doi:10.1001/jama.2019.7525)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Association of Disease Definition, Comorbidity, Prognosis With Probability of Hip Fracture in Older Women
JAMA Internal Medicine
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JUNE 17, 2019
Media advisory: The full study and commentary are linked to this news release.
Embed this link to provide your readers free access to the full-text article This link will be live at the embargo time: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2735986?guestAccessKey=5accbb4f-661a-48b8-99b7-ba81f3d4f915&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=061719
Authors: Members of the Study of Osteoporotic Fractures Research Group; Kristine E. Ensrud, M.D., M.P.H., of the University of Minnesota in Minneapolis, is the corresponding author.
(doi:10.1001/jamainternmed.2019.0682)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Industry Payments to Cath Lab Directors of Top U.S. Hospitals
JAMA Internal Medicine
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JUNE 17, 2019
Media advisory: The full study and Editor’s Note are linked to this news release.
Embed this link to provide your readers free access to the full-text article This link will be live at the embargo time: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2735984?guestAccessKey=d78f6def-8dcb-4ae1-998d-513ce5319b6d&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=061719
Authors: Jeptha P. Curtis, M.D., of the Yale School of Medicine in New Haven, Connecticut, is the corresponding author.
(doi:10.1001/jamainternmed.2018.8775)
Editor’s Note: The article includes conflict of interest disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Youth, Adult Arrests for Cannabis Possession After Decriminalization, Legalization
JAMA Pediatrics
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JUNE 17, 2019
Media advisory: The full study and editorial are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamapediatrics/fullarticle/2735638?guestAccessKey=5b64eaf0-a26a-479e-8b1b-09fef701640f&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=061719
Authors: Andrew D. Plunk, Ph.D., M.P.H., at Eastern Virginia Medical School in Norfolk, is the corresponding author.
(doi:10.1001/jamapediatrics.2019.1539)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Changes in Obesity Among Low-Income Children Enrolled in WIC
JAMA
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, JUNE 18, 2019
Media advisory: To contact corresponding author Liping Pan, M.D., M.P.H., email CDC Media Relations at media@cdc.gov. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jama/fullarticle/2735808?guestAccessKey=cd36d614-7417-494f-95eb-fc10de564fbf&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=061819
Bottom Line: This study looked at changes in overweight and obesity among low-income young children enrolled in the food assistance Special Supplemental Nutrition Program for Women, Infants and Children (WIC) from 2010 to 2016. The analysis included 12.4 million children 2 to 4 years old. Obesity declined between 2010 and 2016 to 13.9% from 15.9%; overweight and obesity combined declined to 29.1% from 32.5%. Declines were seen overall and in all age, sex and racial/ethnic subgroups. Reasons for the declines are unknown but could include WIC food package revisions, along with local, state and national initiatives. A limitation of the study is that fewer children were enrolled in WIC in recent years.
Authors: Liping Pan, M.D., M.P.H., Centers for Disease Control and Prevention, Atlanta, and coauthors
(doi:10.1001/jama.2019.5051)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Study Compares Cognitive Outcomes in Patients With MS Based on Disease Onset
JAMA Neurology
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JUNE 17, 2019
Media Advisory: To contact corresponding author Jan Hillert, M.D., Ph.D., email jan.hillert@ki.se. The full study and editorial are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamaneurology/fullarticle/2735956?guestAccessKey=dcc6137c-8f6f-4aee-98f5-2d409c76555a&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=061719
Bottom Line: Adults who had pediatric-onset of multiple sclerosis (MS) before they were 18 were more likely to have greater cognitive consequences than patients who developed MS as adults. This study used Swedish registry data and included 5,704 patients with MS (300 of whom had pediatric-onset of the disease), and it compared test scores reflective of information-processing efficiency. Researchers report scores were lower, and declined faster, among patients with pediatric-onset MS compared to patients with adult-onset MS. Additionally, patients with pediatric-onset MS were more likely to experience cognitive impairment. Study limitations include misclassification of patients because the date of MS onset was largely based on self-reported symptoms.
Authors: Jan Hillert, M.D., Ph.D., Karolinska Institutet, Stockholm, Sweden, and coauthors
(doi:10.1001/jamaneurol.2019.1546)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Is Sexting Associated With Sexual Behaviors, Mental Health Among Teens?
JAMA Pediatrics
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JUNE 17, 2019
Media advisory: To contact corresponding author Sheri Madigan, Ph.D., email Heath McCoy at hjmccoy@ucalgary.ca. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamapediatrics/fullarticle/2735639?guestAccessKey=0d6a6da6-54f2-454c-a9ab-ef9e718d726f&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=061719
Bottom Line: This study, called a systematic review and meta-analysis, combined the results of 23 studies with nearly 42,000 participants to summarize associations between sexting by adolescents, sexual behavior and mental health risk factors. The results suggest sexting was associated with sexual activity, multiple sex partners, a lack of contraception use, delinquent behavior, anxiety/depression, alcohol and drug use, and smoking. Some associations between sexting, sexual behaviors and mental health factors were stronger in younger compared with older adolescents. This kind of study can only suggest correlations, not causation, and other variables, such as intention and context, can mediate the observed associations. For example, sexual exploration among adolescents is normal, sexting can have relatively harmless intentions and sexting within the context of a romantic relationship may not indicate risky behavior. That’s why more research is needed to understand what the intersections of sexting, sexual behaviors and mental health risk factors mean.
Author: Sheri Madigan, Ph.D., of the Unversity of Calgary, Canada, and coauthors
(doi:10.1001/jamapediatrics.2019.1658)
Editor’s Note: The article contains funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Suicide Rates Among U.S. Adolescents, Young Adults Continue to Increase
JAMA
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, JUNE 18, 2019
Media advisory: To contact corresponding author Oren Miron, M.A., email Ekaterina Pesheva at Ekaterina_Pesheva@hms.harvard.edu. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jama/fullarticle/2735809?guestAccessKey=04de2fe2-1b68-4ad8-9afb-e5196b877b2b&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=061819
Bottom Line: A detailed analysis of recent national data on suicide rates among young people ages 15 to 24 reports 6,241 suicides in 2017, and suicide rates at ages 15 to 19 and 20 to 24 that have increased to their highest point since 2000. This study used data from the Centers for Disease Control and Prevention to take a closer look at suicide rates in the United States among young people and to see if increases from 2000 to 2016 were continuing. Of the 6,241 suicides in 2017, 5,016 were male and 1,225 were female; the suicide rate for those 15 to 19 was 11.8 per 100,000 compared with 8 per 100,000 in 2000. For young adults 20 to 24, the suicide rate in 2017 was 17 per 100,000 compared with 12.5 per 100,000 in 2000. A limitation of the study is that causes of death in death certificates can sometimes be wrong, for example, if a suicide using opioids is mistaken for an accidental overdose. The observed increase in suicide also could reflect better reporting. Future studies should examine other possible contributing factors to the increasing suicide rate.
Authors: Oren Miron, M.A., Harvard Medical School, Boston, and coauthors
(doi:10.1001/jama.2019.5054)
Editor’s Note: The article contains funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Suicide Among Older Adults In, Transitioning to Residential Long-Term Care
JAMA Network Open
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, JUNE 14, 2019
Media advisory: To contact corresponding author Briana Mezuk, Ph.D., at the University of Michigan School of Public Health, Ann Arbor, email Andrea LaFerle at alaferle@umich.edu. The full study and commentary are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2019.5627?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=061419
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
(doi:10.1001/jamanetworkopen.2019.5627)
Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
How Was Medicaid Expansion Associated With Rates of Child Maltreatment?
JAMA Network Open
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, JUNE 14, 2019
Media advisory: To contact corresponding author Emily C.B.Brown, M.D., M.S., email press@seattlechildrens.org. The full study and commentary are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2019.5529?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=061419
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: State-level data were analyzed to determine whether Medicaid expansion was associated with changes in rates of physical abuse and neglect of children younger than 6. Medicaid expansion was part of the the Affordable Care Act and prior research suggests it was associated with better financial stability for families and parents’ access to mental health care, both risk factors for child maltreatment. This study included data from 2010 through 2016 for 31 states and the District of Columbia that expanded Medicaid and 19 states that didn’t. Medicaid expansion was associated with a reduction in the reported child neglect rate (422 fewer cases of reported neglect per 100,000 children younger than 6) but not with a significant change to the physical abuse rate, which authors suggest truly may not be there but also could reflect a delayed association not captured by the short study period. Medicaid varies by state and the study may not have captured the effects of program variations that may have affected the association with child maltreatment. Future research should focus on understanding the reasons behind these study findings.
Authors: Emily C.B.Brown, M.D., M.S., of Seattle Children’s Hospital, and coauthors
(doi:10.1001/jamanetworkopen.2019.5529)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Exposure to Artificial Light At Night While Sleeping and Women’s Weight
JAMA Internal Medicine
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JUNE 10, 2019
Media advisory: To contact corresponding authors Dale P. Sandler, Ph.D., and Yong-Moon Mark Park, M.D., Ph.D., email Robin Arnette at arnetter@niehs.nih.gov. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2735446?guestAccessKey=5a107249-1470-48f6-a8fe-ffe680065c54&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=061019
Bottom Line: Exposure to artificial light at night, especially sleeping with a light or television on in the room, was associated with increased risk of weight gain and overweight and obesity among a large group of women studied. However, researchers were quick to point out that exposure to artificial light at night can be indicative of socioeconomic disadvantage or unhealthy behaviors, which could contribute to weight gain and obesity. This observational study included nearly 44,000 women in its analysis. The women, who were enrolled in the Sister Study group, had no history of cancer or cardiovascular disease and weren’t shift workers, daytime sleepers or pregnant at the study’s start. Exposure to any artificial light at night while sleeping was associated with measures of obesity at the study baseline and follow-up. Exposure to artificial light at night was self-reported. Although causal inferences cannot be drawn from these results and more studies are needed to examine this association, reducing exposure to artificial light at night while sleeping could be considered in obesity prevention interventions.
Authors: Dale P. Sandler, Ph.D., and Yong-Moon Mark Park, M.D., Ph.D., of the National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, North Carolina, and coauthors
(doi:10.1001/jamainternmed.2019.0571)
Editor’s Note: The article includes a funding/support disclosure. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Are Blood Donor Sex, Pregnancy History and Death of Transfusion Recipients Associated?
JAMA
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, JUNE 11, 2019
Media advisory: To contact corresponding author Gustaf Edgren, M.D., Ph.D., email Gustaf.Edgren@ki.se. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jama/fullarticle/2735501?guestAccessKey=dbd8ae43-82a7-4b63-9cba-cdc7ff4aa291?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=061119
Bottom Line: Whether blood donors’ sex and pregnancy history were associated with death for red blood cell transfusion recipients was investigated in this study that analyzed data from three study groups totaling more than 1 million transfusion recipients. There were no statistically significant associations between any of the three donor characteristics studied (female donors, previously pregnant donors, and donors and recipients who were of opposite sex) with in-hospital mortality of transfusion recipients in any of the three study groups. Prior research has produced conflicting results about possible associations between red blood cells from female donors and increased risk of death for transfusion recipients. The study has several limitations, including its observational design which can only show associations.
Authors: Gustaf Edgren, M.D., Ph.D., Karolinska Institutet, Stockholm, Sweden, and coauthors
(doi:10.1001/jama.2019.7084)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
USPSTF Recommendation on Screening for HIV Infection
JAMA
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, JUNE 11, 2019
Media advisory: To contact the U.S. Preventive Services Task Force, email the Media Coordinator at Newsroom@USPSTF.net or call 202-572-2044. The full report, related articles and a podcast are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time and all USPSTF articles remain free indefinitely: https://jamanetwork.com/journals/jama/fullarticle/2735345?guestAccessKey=bbd459cf-657e-4928-bacc-b01f1bdbe7ea&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=061119
Bottom Line: The U.S. Preventive Services Task Force (USPSTF) recommends screening for HIV infection in adolescents and adults ages 15 to 65; in those younger or older at increased risk of infection; and in all pregnant people. The USPSTF routinely makes recommendations about the effectiveness of preventive care services and this statement is an update of its 2013 recommendation. About 15% of people living with HIV are unaware of their infection, and it is estimated that those individuals are responsible for 40% of HIV transmissions in the United States. There were about 38,000 new cases of HIV infection in 2017.
The USPSTF Concludes:

(doi:10.1001/jama.2019.6587)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
Note: More information about the U.S. Preventive Services Task Force, its process, and its recommendations can be found on the newsroom page of its website.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
State Abortion Restrictions, the Supreme Court, and Women’s Access to Reproductive Health Services
JAMA
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, JUNE 5, 2019
Media advisory: To contact authors Lawrence O. Gostin, J.D., and Rebecca B. Reingold, J.D., both of the Georgetown University Law Center, Washington, D.C., email Karen Teber at km463@georgetown.edu. The Viewpoint is linked to this news release and a table of state abortion laws is attached in a PDF below.
Embed this link to provide your readers free access to the full-text article This link will be live at the embargo time: https://jamanetwork.com/journals/jama/fullarticle/2735678?guestAccessKey=76276d02-a8cb-4b7a-8382-ac6e97a42596&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=060519
Supplemental Content: Table of state abortion laws. Click on this link to download a PDF of the table: 06-05 JAMA abortion law supp
(doi:10.1001/jama.2019.8437)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
USPSTF Recommends PrEP to Prevent HIV Infection in People at High Risk
JAMA
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, JUNE 11, 2019
Media advisory: To contact the U.S. Preventive Services Task Force, email the Media Coordinator at Newsroom@USPSTF.net or call 202-572-2044. The full report, related articles and a podcast are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time and all USPSTF articles remain free indefinitely: https://jamanetwork.com/journals/jama/fullarticle/2735509?guestAccessKey=e7dfb28b-1d9c-4351-ba42-964c790a443f&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=061119
Bottom Line: In a new recommendation, the U.S. Preventive Services Task Force (USPSTF) recommends clinicians offer preexposure prophylaxis (PrEP) with effective antiretroviral therapy to people at high risk of acquiring HIV to decrease their risk of infection with the virus that causes AIDS. The USPSTF routinely makes recommendations about the effectiveness of preventive care services. This recommendation statement comes after a review of the evidence on the benefits of PrEP to prevent HIV infection. There were more than 38,000 new diagnoses of HIV infection reported in the United States in 2017. HIV is now treatable but there is no cure and the virus can have significant health consequences.
The USPSTF Concludes:

(doi:10.1001/jama.2019.6390)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
Note: More information about the U.S. Preventive Services Task Force, its process, and its recommendations can be found on the newsroom page of its website.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Firearms and Risk of Suicide by U.S. Army Soldiers
JAMA Network Open
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, JUNE 7, 2019
Media advisory: To contact corresponding author Catherine L. Dempsey, Ph.D., email Sarah Marshall at sarah.marshall@usuhs.edu. The full study and commentary are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2019.5383?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=060719
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: Examining to what extent firearm ownership and accessibility may be associated with suicide risk among U.S Army soldiers was the aim of this psychological autopsy study. The study included 135 soldiers who died by suicide on active duty between 2011-2013; firearms were the most common method with 61 of 111 soldiers who had a documented method of injury dying this way. Other soldiers were included for comparison based on sociodemographic and Army history risk factors for suicide death, as well as soldiers with recent suicidal thoughts. Next-of-kin and Army supervisors were interviewed about soldiers who died by suicide. Soldiers who died by suicide were more likely to own firearms, store loaded firearms at home, and carry personal guns in public than some other soldiers, and those factors combined were associated with an increased likelihood of suicide death. The study was limited by the small number of soldiers included. The results suggest a continued focus on lethal means counseling is warranted and one aspect of focus could be the separate storage of ammunition and firearms.
Authors: Catherine L. Dempsey, Ph.D., Uniformed Services University of the Health Sciences, Bethesda, Maryland, and coauthors
(doi:10.1001/jamanetworkopen.2019.5383)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Contributors to Postinjury Mental Health in Urban Black Men With Serious Injuries
JAMA Surgery
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, JUNE 5, 2019
Media advisory: To contact corresponding author Therese S. Richmond, Ph.D., C.R.N.P., of the University of Pennsylvania, Philadelphia, email media@nursing.upenn.edu. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamasurgery/fullarticle/2735117?guestAccessKey=3e0fe88f-3303-4fad-8fd8-37083b7bb39f&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=060519
(doi:10.1001/jamasurg.2019.1622)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.)
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Association of Antiviral Therapy With Parkinson Disease Risk in Hepatitis C Infection
JAMA Neurology
EMBARGOED FOR RELEASE: 10:30 P.M. (ET), WEDNESDAY, JUNE 5, 2019
Media Advisory: To contact the corresponding authors, email Ying-Zu Huang, M.D., Ph.D., at yzhuang@cgmh.org.tw and Rou-Shayn Chen, M.D., at cerebrum@cgmh.org.tw, both of the Chang
Gung Memorial Hospital, Linkou Medical Center, Taoyuan, Taiwan. The full study and editorial are linked to this news release. This article is being released to coincide with presentation at a meeting.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamaneurology/fullarticle/2735574?guestAccessKey=953e3713-30af-4d14-af58-e7dee358d6e9&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=060519
(doi:10.1001/jamaneurol.2019.1368)
Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Availability of Opioid-Overdose Antidote at Pharmacies
JAMA Network Open
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, JUNE 7, 2019
Media advisory: To contact corresponding author Dima M. Qato, Pharm.D., M.P.H., Ph.D., email Jacqueline Carey at jmcarey@uic.edu. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2019.5388?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=060719
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: Pennsylvania became one of the first states in 2015 to implement a statewide standing order allowing pharmacists to dispense naloxone, an antidote for opioid overdoses, without a physician’s prescription. This study looked at the availability (with or without a prescription) of naloxone nasal spray at 418 pharmacies in Philadelphia surveyed by telephone. The authors report 1 in 3 pharmacies had naloxone nasal spray in stock but many still required a physician’s prescription. Naloxone also was more likely to be in stock and available without a prescription at chain stores than independent ones but naloxone was less likely to be available in areas with elevated rates of opioid overdose deaths. Researchers examined only the availability of naloxone as a nasal spray, and not in other forms, which may have underestimated its availability. The findings suggest efforts are needed to strengthen the implementation of statewide standing orders for the availability of naloxone.
Authors: Dima M. Qato, Pharm.D., M.P.H., Ph.D., University of Illinois at Chicago, and coauthors
(doi:10.1001/jamanetworkopen.2019.5388)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Medicaid Expansion Associated With Fewer Cardiovascular Deaths
JAMA Cardiology
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, JUNE 5, 2019
Media advisory: To contact corresponding author Sameed Ahmed M. Khatana, M.D., email Mike Iorfino at Mike.Iorfino@pennmedicine.upenn.edu. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article This link will be live at the embargo time: https://jamanetwork.com/journals/jamacardiology/fullarticle/2734704?guestAccessKey=45939c4b-f955-445e-a181-2cc69c907e47&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=060519
Bottom Line: Expanding Medicaid eligibility was associated with lower rates of death from cardiovascular causes in a study comparing data from counties in 29 states that expanded Medicaid with 19 states that didn’t from 2010 to 2016. Cardiovascular mortality among adults ages 45 to 64 was stable in counties in expansion states between the preexpansion and postexpansion periods (146.5 to 146.4 deaths per 100,000 residents per year). In counties in nonexpansion states, there was an increase in cardiovascular mortality rates during the preexpansion and postexpansion periods (176.3 to 180.9 deaths per 100,000 residents per year). Counties in expansion states had 4.3 fewer deaths from cardiovascular causes per 100,000 residents per year after accounting for differences in demographic, clinical, economic and health access factors. This is an observational study so causal inferences can’t be drawn between the expansion of Medicaid eligibility and differences in cardiovascular mortality rates. Policymakers may want to consider these results as they debate more changes to Medicaid eligibility and expansion.
Authors: Sameed Ahmed M. Khatana, M.D., University of Pennsylvania, Philadelphia, and coauthors
(doi:10.1001/jamacardio.2019.1651)
Editor’s Note: The article contains conflict of interest disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Association of SC’s Medicaid Payment Changes With Immediate Postpartum Long-Acting Contraception, Birth Intervals
JAMA
EMBARGOED FOR RELEASE: 5 P.M. (ET), MONDAY, JUNE 3, 2019
Media advisory: To contact corresponding author Maria W. Steenland, S.D., M.P.H., at Brown University, Providence, Rhode Island, email Mollie Rappe at mollie_rappe@brown.edu. The full study is linked to this news release. This article is being released to coincide with a meeting presentation.
Embed this link to provide your readers free access to the full-text article This link will be live at the embargo time: https://jamanetwork.com/journals/jama/fullarticle/2735316?guestAccessKey=4a56330e-2909-49f9-91e5-96b6c568e60d&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=060319
(doi:10.1001/jama.2019.6854)
Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Effect of Early Limited Formula, Breastfeeding Duration in the 1st Year
JAMA Pediatrics
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JUNE 3, 2019
Media advisory: To contact corresponding author Valerie J. Flaherman, M.D., M.P.H., of the University of California, San Francisco, email Suzanne Leigh at Suzanne.Leigh@ucsf.edu. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamapediatrics/fullarticle/2734206?guestAccessKey=9c994f33-1ba8-4ff1-8b0b-300ee0a7fd5f&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=060319
(doi:10.1001/jamapediatrics.2019.1424)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Study Looks at Path to Recovery of Full Daily Function After Mild TBI
JAMA Neurology
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JUNE 3, 2019
Media Advisory: To contact corresponding author Lindsay D. Nelson, Ph.D., email Holly Botsford at hbotsford@mcw.edu. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamaneurology/fullarticle/2735106?guestAccessKey=29b4cd1f-dbea-4abc-8eda-3da4c792b87c&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=060319
Bottom Line: This study describes the path to recovery of daily function in patients with mild traumatic brain injury (mTBI) using data from a study that followed a group of patients with mTBI over time. The study included 1,154 patients with mTBI who sought care at level 1 trauma centers and 299 patients with orthopedic injuries but no signs of head trauma for comparison. The two groups of patients weren’t statistically significantly different postinjury from two weeks to six months based on reported functional limitations. However, at 12 months postinjury more patients in the mTBI group continued to report limitations compared to those patients in the group with orthopedic injuries. At 12 months, 47% of patients with mTBI but 62% of those with orthopedic injuries reported a full return to their preinjury functioning levels. The results of this observational study may not be generalizable to a broader population of patients with mTBI, including those who didn’t go to emergency departments. Also, the assessment to measure aspects of daily functioning is completed through interviews with patients or their proxies. These findings suggest the need for more follow-up of patients after mTBI to reduce the chance of chronic problems after injury.
Authors: Lindsay D. Nelson, Ph.D., of the Medical College of Wisconsin, Milwaukee, and coauthors
(doi:10.1001/jamaneurol.2019.1313)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Care Cascades After Low-Value Preoperative Testing
JAMA Internal Medicine
EMBARGOED FOR RELEASE: 3 P.M. (ET), MONDAY, JUNE 3, 2019
Media advisory: To contact corresponding author Ishani Ganguli, M.D., M.P.H., of Brigham and Women’s Hospital, Boston, email Elaine St. Peter at estpeter@bwh.harvard.edu. The full study is linked to this news release. This article is being released to coincide with a meeting presentation.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2735387?guestAccessKey=ec4953d8-8bf8-48af-b397-b7ceebfe191e&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=060319
(doi:10.1001/jamainternmed.2019.1739)
Editor’s Note: The articles conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanet
How Common is Nonsuicidal Self-injury Among Sexual Minority, Heterosexual Adolescents?
JAMA Pediatrics
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, JUNE 3, 2019
Media advisory: To contact corresponding author Richard T. Liu., Ph.D., email Mollie Rappe at mollie_rappe@brown.edu. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamapediatrics/fullarticle/2734202?guestAccessKey=7b4babb1-80e1-4c57-8117-7fb99be5d6fd&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=060319
Bottom Line: This study describes how common nonsuicidal self-injury has been over time among sexual minority and heterosexual adolescents. The study used surveillance data for 2005 to 2017 from Massachusetts, the first state to assess sexual orientation (assessed as self-reported sexual identity and same-sex behavior). Among about 21,200 adolescents in grades 9 through 12, rates of nonsuicidal self-injury ranged from 11% to 20% among heterosexual young people and from 38% to 53% among sexual minority youth across the study period. Rates may be even higher because of how nonsuicidal self-injury was measured. There is a pressing concern for progress in addressing this public health concern.
Author: Richard T. Liu, Ph.D., Alpert Medical School of Brown University, Providence, Rhode Island.
(doi:10.1001/jamapediatrics.2019.1433)
Editor’s Note: The article contains funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Deaths From Falls Increase Among Older U.S. Adults
JAMA
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, JUNE 4, 2019
Media advisory: To contact corresponding author Klaas A. Hartholt, M.D., Ph.D., email k.hartholt@rdgg.nl. The full study, editorial and related articles are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jama/fullarticle/2735063?guestAccessKey=df56bbcc-17ca-47b0-986f-77bf6d03962e&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=060419
Bottom Line: Death rates from falls for U.S. adults 75 or older increased from 2000 to 2016 in this study that analyzed nationally representative vital statistics data. The absolute number of deaths from falls increased from 8,613 in 2000 to 25,189 in 2016. The overall rate of death increased from nearly 52 per 100,000 people in 2000 to 122 per 100,000 in 2016. Rates of death from falls adjusted for different age distributions increased from about 61 per 100,000 men in 2000 to about 116 per 100,000 in 2016; among women rates increased from 46 per 100,000 in 2000 to about 106 per 100,000 in 2016. Rates of death increased by age group in 2016 from 42 per 100,000 among those ages 75 to 79 compared with about 591 per 100,000 among those 95 or older. The reasons for these increases aren’t fully known, although misclassification or incomplete recording of causes of death could have resulted in overestimation or underestimation of deaths from falls.
Authors: Klaas A. Hartholt, M.D., Ph.D., Reinier de Graaf Groep, Delft, the Netherlands, and coauthors
(doi:10.1001/jama.2019.4185)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Do Violent Video Games Affect Kids’ Behavior With Real Guns?
JAMA Network Open
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, MAY 31, 2019
Media advisory: To contact authors Brad J. Bushman, Ph.D., and Justin H. Chang, M.A., email Misti L. Crane at crane.11@osu.edu. The full study, commentary and a visual abstract are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2019.4319?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=053119
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: This randomized clinical trial in a university laboratory examined the effects of video games with weapons on children’s behavior when they found a real gun. Pairs of children (ages 8 to 12) were assigned to 1 of 3 versions of the popular video game Minecraft (one child played while the other watched): (1) violent with guns used to kill monsters, (2) violent with swords used to kill monsters or (3) nonviolent with no weapons or monsters. After 20 minutes of gameplay, the children played with other toys in another room that included a cabinet with two disabled handguns. The study analysis included 220 children (average age 10) who found a gun while playing. Nearly 62% of the 76 children who played the video game with gun violence touched a handgun; about 57% of the 74 children who played the game with sword violence touched a gun, and about 44% of the 70 children who played the nonviolent version touched a gun, although the differences across groups weren’t significant. Children exposed to violent versions of the video game were more likely to engage in the dangerous behavior of pulling the trigger at themselves or their partner than children exposed to the nonviolent version. The violent versions with guns and swords were significant even after accounting for other mitigating factors (sex, age, trait aggressiveness, exposure to violent media, attitudes toward guns, presence of firearms in the home, interest in firearms and whether the child had taken a firearm safety course). The other outcomes (time spent holding a gun and total trigger pulls) weren’t statistically significant. Self-reported consumption of violent media was also a risk for total trigger pulls and trigger pulls at self or partner. The study is limited by the artificial setting of a university laboratory and Minecraft is not a very violent game with no blood and gore (researchers could not ethically expose children to a more violent, age-inappropriate game). The authors encourage gun owners to secure their firearms and reduce children’s exposure to violent video games.
Authors: Brad J. Bushman, Ph.D., and Justin H. Chang, M.A., at Ohio State University, Columbus
Visual Abstract:

(doi:10.1001/jamanetworkopen.2019.4319)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Study Examines Youth Suicides After ’13 Reasons Why’
JAMA Psychiatry
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, MAY 29, 2019
Media advisory: To contact corresponding author Thomas Niederkrotenthaler, M.D., Ph.D., M.M.Sc., email Thorsten Medwedeff at thorsten.medwedeff@meduniwien.ac.at or Jakob Sonnleithner at jakob.sonnleithner@meduniwien.ac.at. The full study and editorial are linked to this news release.
Embed this link to provide your readers free access to the full-text article This link will be live at the embargo time: https://jamanetwork.com/journals/jamapsychiatry/fullarticle/2734859?guestAccessKey=dd50899b-1af3-464a-af48-050ff6b6139d&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=052919
Bottom Line: The popular Netflix series “13 Reasons Why” was controversial for its portrayal of the suicide of a 17-year-old girl. This study, called a time series analysis, used suicide data before and after the show’s release in 2017 to estimate suicides among different age groups (10 to 19, 20 to 29, and 30 or older for females and males in the U.S.) and to identify changes in the specific methods of suicide used. The authors report an estimated 13% increase in suicide among individuals 10 to 19 in the three months after the show’s release, which corresponds to 94 more suicides than would be expected. Gender-specific analyses suggest larger proportional increases among females. No similar increase in suicide was seen among the other age groups. The results of this study should be interpreted with caution, in part because the study was based on data that makes it impossible to know whether people who died from suicide had actually watched the show. However, the results point to the need for public health and suicide experts to work with the entertainment industry to prevent harmful portrayals of suicide. Individuals thinking about suicide or worried about someone else can call the National Suicide Prevention Lifeline at 1-800-273-8255.
Authors: Thomas Niederkrotenthaler, M.D., Ph.D., M.M.Sc., of the Medical University of Vienna, Center for Public Health, Vienna, Austria, and coauthors
(doi:10.1001/jamapsychiatry.2019.0922)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Free full-text link: Association of Steps With Death Rates in Women
JAMA Internal Medicine
EMBARGOED FOR RELEASE: 1:55 P.M. (ET), WEDNESDAY, MAY 29, 2019
Media advisory: To contact corresponding author I-Min Lee, M.B.B.S., Sc.D., email Elaine St. Peter at estpeter@bwh.harvard.edu. The full study is linked to this news release. This is being released to coincide with a meeting presentation.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2734709?guestAccessKey=c46927fc-b021-43a6-a43d-26eb90ee54b7&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=052919
(doi:10.1001/jamainternmed.2019.0899)
Editor’s Note: The articles conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanet
Perceived Discrimination Associated With Well-Being in Adults With Poor Vision
JAMA Ophthalmology
EMBARGOED FOR RELEASE: 11 A.M. (ET), THURSDAY, MAY 30, 2019
Media advisory: To contact corresponding author Sarah E. Jackson, Ph.D., email s.e.jackson@ucl.ac.uk. The full study and commentary are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamaophthalmology/fullarticle/2734217?guestAccessKey=f3cab9a2-8e11-40b7-9fc0-275efbc81eea&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=053019
Bottom Line: This study of nearly 7,700 men and women 50 or older in England looked at how common perceived discrimination was among those with visual impairment and how that was associated with emotional well-being. Of the individuals, 913 reported poor overall eyesight and 658 reported poor eyesight up close. Discrimination was more commonly reported by those adults with poor eyesight than those with good eyesight, with the most common form of discrimination reported being treated with less respect or courtesy. Individuals who reported poor eyesight and discrimination were more likely to report depressive symptoms and loneliness, as well as lower quality of life and life satisfaction than those with poor eyesight but no reported perceived discrimination. A limitation of the study is that poor eyesight was self-reported. Health care practitioners should consider asking patients with visual impairment about their well-being.
Authors: Sarah E. Jackson, Ph.D., University College London, and coauthors
(doi:10.1001/jamaophthalmol.2019.1230)
Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Free full-text link: Rx Filling Among Teens Prescribed for STIs in Emergency Departments
JAMA Pediatrics
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, MAY 28, 2019
Media advisory: To contact corresponding author Monika K. Goyal, M.D., M.S.C.E., at Children’s National Health System, Washington, D.C., email media@childrensnational.org. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamapediatrics/fullarticle/2734737?guestAccessKey=ce0ca5b3-e003-4de3-b354-4831844a8324&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=052819
(doi:10.1001/jamapediatrics.2019.1263)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Association of Team Sports in Adolescence, Adult Mental Health
JAMA Pediatrics
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, MAY 28, 2019
Media advisory: To contact corresponding author Molly C. Easterlin, M.D., email Enrique Rivero at erivero@mednet.ucla.edu. The full study and editorial are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamapediatrics/fullarticle/2734743?guestAccessKey=130f8a26-ebd3-4f46-85ee-269ae01e223d&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=052819
Bottom Line: Participation in team sports as an adolescent was associated with a higher likelihood of some better adult mental health outcomes among individuals with adverse childhood experiences (ACES). This observational study included nearly 9,700 individuals with data on their exposure to ACEs, including physical and sexual abuse, emotional neglect, parental alcohol misuse, parental incarceration and living with a single parent. About 49% of the individuals reported exposure to one ACE and about 21% reported two or more ACEs. Team sports participation during adolescence was associated with a lower likelihood of a diagnosis of depression and anxiety in young adulthood but not associated at a statistically significant level with current depressive symptoms after accounting for other mitigating factors. The association between team sports and better mental health wasn’t significantly different for males and females. The study cannot determine causality and social determinants of health are hard to isolate. Team sports may help to mitigate the effects of ACEs but other factors may too. The authors suggest teams sports may help to build resilience so policymakers and child health advocates may want to advocate for these programs to be accessible and equitable.
Authors: Molly C. Easterlin, M.D., of the University of California, Los Angeles, National Clinician Scholars Program, and coauthors
(doi:10.1001/jamapediatrics.2019.1212)
Editor’s Note: The article contains funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org
What is Association Between Early-Onset Alzheimer Disease, Cholesterol Levels?
JAMA Neurology
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, MAY 28, 2019
Media Advisory: To contact corresponding author Thomas S. Wingo, M.D., email Jennifer Johnson at jrjohn9@emory.edu. The full study and editorial are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamaneurology/fullarticle/2734865?guestAccessKey=427f1c57-21e5-4303-9b41-bc3b34156096&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=052819
Bottom Line: This case series study investigated whether cholesterol level was associated with early-onset Alzheimer disease (AD) and what underlying genetic variations there might be. Blood samples were used from 2,125 individuals (654 patients with early-onset AD with symptoms beginning at age 65 or younger and the rest cognitively normal individuals over the age of 60 for comparison). The authors report elevated levels of low-density lipoprotein cholesterol (the so-called “bad” cholesterol) were associated with a higher likelihood of having early-onset AD, and that variations in a gene known to influence cholesterol levels, APOB, were associated too. The gene variations were rare and the small number of cholesterol measurements don’t allow for a causal interpretation of these findings.
Authors: Thomas S. Wingo, M.D., Emory University School of Medicine, Atlanta, and coauthors
(doi:10.1001/jamaneurol.2019.0648)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Free full-text link: Opioid Prescribing by Dentists
JAMA Network Open
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, MAY 24, 2019
Media advisory: To contact corresponding author Katie J. Suda, Pharm.D., M.S., of the Universoty of Illinois at Chicago, email Jacqueline Michelle Carey at jmcarey@uic.edu. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2019.4303?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=052419
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
(doi:10.1001/jamanetworkopen.2019.4303)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelation
Free full-text link: Is Life Purpose Associated With Death?
JAMA Network Open
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, MAY 24, 2019
Media advisory: To contact corresponding author Celeste Leigh Pearce, Ph.D., M.P.H., at the University of Michigan, Ann Arbor, email Andrea LaFerle at alaferle@umich.edu. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2019.4270?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=052419
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
(doi:10.1001/jamanetworkopen.2019.4270)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Free full-text link: Death Rates Among NFL, MLB Players
JAMA Network Open
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, MAY 24, 2019
Media advisory: To contact corresponding author Marc G. Weisskopf, Ph.D., Sc.D., of the Harvard T.H. Chan School of Public Health, Boston, email Christopher Sweeney at csweeney@hsph.harvard.edu. The full study and commentary are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2019.4223?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=052419
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
(doi:10.1001/jamanetworkopen.2019.4223)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Clinical Trial Examines Approaches to Improve Quality of Life for Patients With Tinnitus
JAMA Otolaryngology-Head & Neck Surgery
EMBARGOED FOR RELEASE: 11 A.M. (ET), THURSDAY, MAY 23, 2019
Media advisory: To contact corresponding author Roberta W. Scherer, Ph.D., email Jon Eichberger at je@jhu.edu. The full study, commentary, Viewpoint and podcast are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamaotolaryngology/fullarticle/2734346?guestAccessKey=7152a774-a3c0-4365-94d0-0a7e52b1a93c&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=052319
Bottom Line: This randomized clinical trial of 151 active-duty and retired military personnel and dependents compared tinnitus retraining therapy (combines counseling and sound masking to reduce patient awareness of tinnitus and the distress it may cause) with standard care (counseling protocol aligned with military care and recommended practice guidelines) for reducing the negative effects of ringing in the ears on quality of life. There is no cure for tinnitus and the aim of treatment is to improve of quality of life.
Authors: Roberta W. Scherer, Ph.D., Johns Hopkins Bloomberg School of Public Health, Baltimore, and coauthors
(doi:10.1001/jamaoto.2019.0821)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Study Examines Association of Being Black With Death from Prostate Cancer
JAMA Oncology
EMBARGOED FOR RELEASE: 11 A.M. (ET), THURSDAY, MAY 23, 2019
Media advisory: To contact corresponding author Daniel E. Spratt, M.D., email Ian Demsky at idemsky@med.umich.edu. The full study and commentary are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamaoncology/fullarticle/2734259?guestAccessKey=7b694ab9-a816-4098-9fba-1062b74757b0&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=052319
Bottom Line: How being black is associated with prostate cancer survival after accounting for nonbiological differences, such as access to care and type of treatment, was the subject of this analysis that included 306,000 men with prostate cancer from three study groups. When black men with nonmetastatic prostate cancer had similar access to care and standardized treatment, they appeared to have comparable prostate cancer-specific mortality to white men, although being black was associated with increased risk of death from other causes. The results of this observational study suggest efforts are needed to address the modifiable social factors that contribute to racial disparities in prostate cancer outcomes. The data don’t address the biological likelihood of developing prostate cancer.
Authors: Daniel E. Spratt, M.D., University of Michigan, Ann Arbor, and coauthors
(doi:10.1001/jamaoncol.2019.0826)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Parental Use of Prescription Opioids Associated With Risk of Suicide Attempt by Children
JAMA Psychiatry
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, MAY 22, 2019
Media advisory: To contact corresponding author Robert D. Gibbons, Ph.D., email Matt Wood at Matthew.Wood@uchospitals.edu. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article This link will be live at the embargo time: https://jamanetwork.com/journals/jamapsychiatry/fullarticle/2733148?guestAccessKey=3e0dab45-7ce5-4900-83dc-b38b9b11825a&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=052219
Bottom Line: Opioid use by parents was associated with increased risk of suicide attempt by their children in a study that linked medical claims for opioid prescriptions for parents with medical claims for suicide attempts by their children. This observational study included 184,000 children whose parents used opioids and about 148,000 children whose parents didn’t. Parental opioid use was defined having filled prescriptions covering more than a year of an opioid between 2010 and 2016. Of the children whose parents didn’t use opioids, 212 (0.14%) attempted suicide, while 678 (0.37%) of the children whose parents used opioids attempted suicide. The increased risk of suicide attempt among children whose parents used opioids remained even after accounting for child age and sex, parent and child depression and substance use diagnoses, and parental history of suicide attempt. Limitations of the study include a conservative definition of parental opioid use and all the claims were for families with private health insurance.
Authors: Robert D. Gibbons, Ph.D., University of Chicago, and coauthors
(doi:10.1001/jamapsychiatry.2019.0940)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Can Blood Donation Programs Identify Donors With Genetic Disorder for High Cholesterol, Coronary Artery Disease?
JAMA Cardiology
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, MAY 22, 2019
Media advisory: To contact corresponding author Amit Khera, M.D., M.Sc., email Lori Soderbergh at Lori.Soderbergh@UTSouthwestern.edu. The full study and commentary are linked to this news release.
Embed this link to provide your readers free access to the full-text article This link will be live at the embargo time: https://jamanetwork.com/journals/jamacardiology/fullarticle/2733135?guestAccessKey=55331a28-fe61-453c-9bb7-038ef98848e2&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=052219
Bottom Line: This study examined whether a blood donation program could help identify individuals with the often undiagnosed genetic disorder familial hypercholesterolemia, which results in high cholesterol and premature coronary artery disease. Blood donation programs already screen for infectious diseases and may be able to screen for other conditions. This analysis included data from about 1.2 million donors who made a total of 3 million blood donations; total cholesterol levels were determined for these donations. The authors report that of these donors, 3,473 (1 of every 339) met the criteria for familial hypercholesterolemia, similar to estimates in the general population, which suggests blood donation programs may be a method for detecting and notifying donors with this disorder. A limitation of the study was the inability to determine whether there were other causes of hypercholesterolemia.
Author: Amit Khera, M.D., M.Sc., University of Texas Southwestern Medical Center, Dallas, and coauthors
(doi:10.1001/jamacardio.2019.1518)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Free full-text link: Some Changes in Air Quality in California Associated With Decrease in Asthma Cases
JAMA
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, MAY 21, 2019
Media advisory: To contact corresponding author Erika Garcia, Ph.D., of the University of Southern California, Los Angeles, email Leigh Hopper at lhopper@usc.edu. The full study and editorial are linked to this news release.
Embed this link to provide your readers free access to the full-text article This link will be live at the embargo time: https://jamanetwork.com/journals/jama/fullarticle/2733972?guestAccessKey=1dd7b496-e0c1-4822-8ea1-b0d2ef03f0f7&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=052119
(doi:10.1001/jama.2019.5357)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Free full-text link: Condom Use With Contraception Among Teen Moms
JAMA Pediatrics
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, MAY 20, 2019
Media advisory: To contact corresponding author Katherine Kortsmit, Ph.D., M.P.H., R.D., of the Centers for Disease Control and Prevention, Atlanta, email Anita Blankenship at aob4@cdc.gov. The full study and editorial are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamapediatrics/fullarticle/2733859?guestAccessKey=e32ec4ee-ff37-40b1-aef0-f8b41140ac5f?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=052019
(doi:10.1001/jamapediatrics.2019.1136)
Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@ja
Free full-text link: Age of JUUL Twitter Followers
JAMA Pediatrics
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, MAY 20, 2019
Media advisory: To contact corresponding author Annice E. Kim, Ph.D., of the Center for Health Policy Science and Tobacco Research, RTI International, Research Triangle Park, North Carolina, email news@rti.org. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamapediatrics/fullarticle/2733855?guestAccessKey=8ee12ee2-ab9f-4d10-a923-2f1c300f1fd0?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=052019
(doi:10.1001/jamapediatrics.2019.0922)
Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@ja
Free full-text link: Suicide Among U.S. Youth
JAMA Network Open
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, MAY 17, 2019
Media advisory: To contact corresponding author Donna A. Ruch, Ph.D., of the Research Institute at Nationwide Children’s Hospital, Columbus, Ohio, email Mary Ellen Fiorino at MaryEllen.Fiorino@nationwidechildrens.org. The full study and commentary are linked to this news release and a podcast is attached.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2019.3886?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=051719
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
(doi:10.1001/jamanetworkopen.2019.3121)
Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@ja
Statin Use Associated With Reduced Risk of Dementia After Concussion in Older Adults
JAMA Neurology
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, MAY 20, 2019
Media Advisory: To contact corresponding author Donald A. Redelmeier, M.D., M.S.H.S.R., email Deborah Creatura at deborah.creatura@ices.on.ca. The full study, editorial and podcast are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamaneurology/fullarticle/2733673?guestAccessKey=bf02d03e-cf9a-4beb-8a91-e70ba3d960c0&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=052019
Bottom Line: Concussion is a common brain injury. This observational study of nearly 29,000 adults (66 and older) diagnosed with concussion examined whether statin use was associated with risk of long-term dementia after a concussion. The analysis compared 7,058 patients who received a statin prescription in the 90 days after a concussion with 21,757 who didn’t. After an average follow-up of four years, 4,727 patients developed dementia (1 in 6). The risk of dementia in older adults after concussion was substantial, and statin use was associated with modestly reduced risk compared to patients who didn’t receive a statin. Limitations of the study include missing information on other factors that could influence the risk of dementia.
Authors: Donald A. Redelmeier, M.D., M.S.H.S.R., University of Toronto, and coauthors
(doi:10.1001/jamaneurol.2019.1148)
Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Free full-text link: Association of Sugary Beverages, Mortality
JAMA Network Open
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, MAY 17, 2019
Media advisory: To contact corresponding author Jean A. Welsh, R.N., M.P.H., Ph.D., of Emory University, Atlanta, email Holly Korschun at hkorsch@emory.edu. The full study and commentary are linked to this news release and a podcast and visual abstract are attached.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2019.3121?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=051719
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Visual Abstract
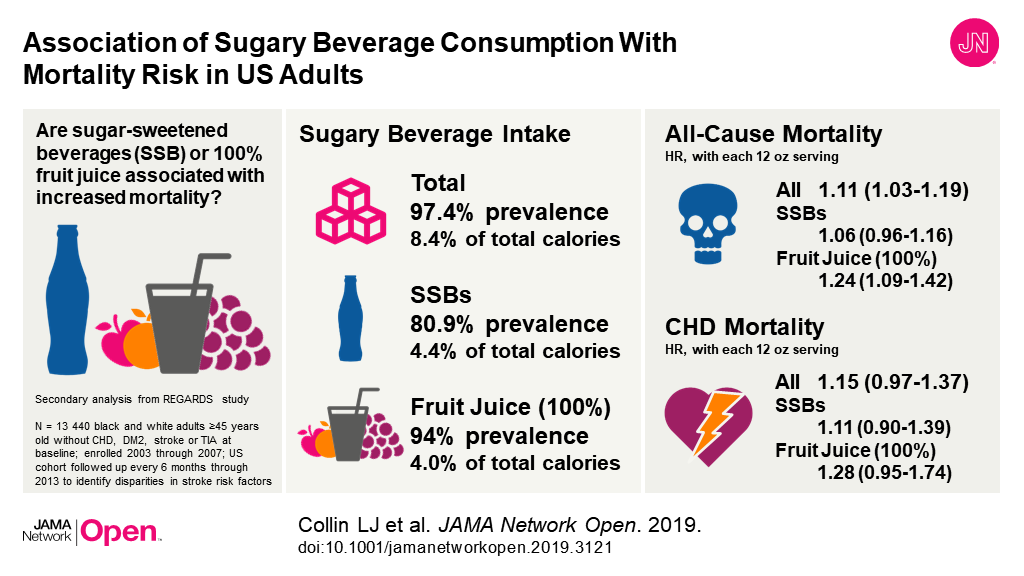
(doi:10.1001/jamanetworkopen.2019.3121)
Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Did Cholesterol Levels Improve Among U.S. Kids, Adolescents?
JAMA
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, MAY 21, 2019
Media advisory: To contact corresponding author Amanda M. Perak, M.D., M.S., email Julie Pesch at jpesch@luriechildrens.org. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jama/fullarticle/2733971?guestAccessKey=97a04b3a-98ba-4c7d-b239-a8a5be2c730b&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=052119
Bottom Line: This study examined cholesterol levels in children and adolescents in the U.S. from 1999 to 2016. Using nationally representative survey data for 26,000 young people (ages 6 to 19), the authors report favorable changes in cholesterol. Average levels of total cholesterol, non-high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides and apolipoprotein B decreased and average levels of the so-called good high-density lipoprotein cholesterol increased. Still, estimates suggest only about half of the participants had ideal levels for all measures and as many as 25% had at least one suboptimal level.
Authors: Amanda M. Perak, M.D., M.S., Ann and Robert H. Lurie Children’s Hospital of Chicago, Northwestern University Feinberg School of Medicine, Chicago, and coauthors
(doi:10.1001/jama.2019.4984)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Free full-text link: Body Compostion, Cardiovascular Events in Patients With Colorectal Cancer
JAMA Oncology
EMBARGOED FOR RELEASE: 11 A.M. (ET), THURSDAY, MAY 16, 2019
Media advisory: To contact corresponding author Justin C. Brown, Ph.D., email Lisa Stansbury at Lisa.stansbury@pbrc.edu. The full study and commentary are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamaoncology/fullarticle/2733794?guestAccessKey=32cd8708-8ee0-4e83-b4fd-3ffa95b74558&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=051619
(doi:10.1001/jamaoncol.2019.0695)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Free full-text link: Association of Vision Impairment With Anxiety, Depression Symptoms
JAMA Ophthalmology
EMBARGOED FOR RELEASE: 11 A.M. (ET), THURSDAY, MAY 16, 2019
Media advisory: To contact corresponding author Joshua R. Ehrlich, M.D., M.P.H., email Kara Gavin at kegavin@med.umich.edu. The full study and commentary are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamaophthalmology/fullarticle/2733451?guestAccessKey=eadf4a9b-b99d-4476-8906-f632164354a4&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=051619
(doi:10.1001/jamaophthalmol.2019.1085)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
End-of-Career Transitions for Older Surgeons
JAMA Surgery
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, MAY 15, 2019
Media advisory: To contact corresponding author Todd K. Rosengart, M.D., email Dipali Pathak at pathak@bcm.edu. The full study, commentary and podcast are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamasurgery/fullarticle/2733041?guestAccessKey=68a225ee-6a6f-43d5-b437-b7676ebcc7f9&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=051519
Bottom Line: A special communication article focuses on end-of-career transitions for older surgeons. The goal is to support an aging workforce while ensuring patient safety.
Authors: Todd K. Rosengart, M.D., Baylor College of Medicine, Houston, and coauthors
(doi:10.1001/jamasurg.2019.1159)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Association Between Benzodiazepine Use in Early Pregnancy, Miscarriage Risk
JAMA Psychiatry
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, MAY 15, 2019
Media advisory: To contact corresponding author Anick Berard, Ph.D., email Florence Meney at Florence.Meney.hsj@ssss.gouv.qc.ca. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamapsychiatry/fullarticle/2733517?guestAccessKey=b19138a8-8ac9-4d51-be62-6e52aaa3fa9b&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=051519
Bottom Line: Benzodiazepines are a class of drugs used to treat anxiety, insomnia and mood disorders. This observational study examined the risk of miscarriage associated with their use in early pregnancy by drug class, specific agent and short- or long-acting formulation in about 442,000 pregnancies in Canada from 1998 through 2015. Among the pregnancies, 27,000 (6.1%) ended in miscarriage and 1.4% of those miscarriages were among women who used benzodiazepines in early pregnancy. The study reports any benzodiazepine use during early pregnancy was associated with increased risk of miscarriage. A limitation of the study is its lack of information on alcohol use by the women, which could have influenced the results. These findings suggest physicians should carefully evaluate the risks and benefits of prescribing benzodiazepines in early pregnancy since alternative treatments exist.
Authors: Anick Berard, Ph.D., Centre Hospitalier Universitaire Sainte-Justine, Montreal, and coauthors
(doi:10.1001/jamapsychiatry.2019.0963)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Is Being Bullied as Teen Associated With Growing Up in Areas of Income Inequality?
JAMA Pediatrics
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, MAY 13, 2019
Media advisory: To contact corresponding author Frank J. Elgar, Ph.D., email Cynthia Lee at cynthia.lee@mcgill.ca. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamapediatrics/fullarticle/2733159?guestAccessKey=5d6b5102-4999-42cd-a9eb-f8d30971dd2c&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=051319
Bottom Line: A survey study of about 874,000 adolescents from 40 European and North American countries suggests growing up in areas with income inequality was associated with being bullied after accounting for some other mitigating factors. The study didn’t contain individial-level or school-level data that might help to explain the observed association with bullying, which was self-reported and uncorroborated. Study authors says more research is needed to understand why children who grow up in economically unequal area may be at greater risk.
Authors: Frank J. Elgar, Ph.D., of McGill University, Montreal, Canada, and coauthors
(doi:10.1001/jamapediatrics.2019.1181)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Summary Video Available
Video embed code:
Texting While Driving Common Among Millennial, Older Parents
JAMA Pediatrics
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, MAY 13, 2019
Media advisory: To contact corresponding author Regan W. Bergmark, M.D., email Elaine St. Peter at estpeter@partners.org.The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamapediatrics/fullarticle/2733153?guestAccessKey=ea4a851a-64e4-4401-a4a5-41c765ad4c21&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=051319
Bottom Line: A distracted driving survey of millennial parents (ages 22 to 37) and older parents (37 and up) shows that most parents had read and written texts while driving in the part 30 days but millennial parents had higher survey scores that reflected more reckless driving behavior, including the use of email, social media and maps plus speed of travel. The survey included 435 parents from 45 states and survey scores were associated with crash rate. This study has limitations, including the potential for inaccurate recollections by parents and a possible bias for more technologically savvy parents because the survey was online. Few parents said their pediatrician had talked to them about distracted driving and few used an app to restrict texting while driving. Both could be potential intervention strategies.
Authors: Regan W. Bergmark, M.D., of Brigham and Women’s Hospital, Harvard Medical School, Boston, and coauthors
(doi:10.1001/jamapediatrics.2019.0830)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Does Fracture Risk Differ Between 2 Common Types of Weight-Loss Surgery?
JAMA Surgery
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, MAY 15, 2019
Media advisory: To contact corresponding author Elaine W. Yu, M.D., M.M.Sc., email Noah Brown at NBROWN9@PARTNERS.ORG. The full study and commentary are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamasurgery/fullarticle/2733040?guestAccessKey=0499b524-760a-493c-803b-b7945323d38b&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=051519
Bottom Line: This study used Medicare claims data to compare risk of fracture among about 42,000 patients who had weight-loss surgery. Nearly 30,000 patients (average age 51) who had Roux-en-Y gastric bypass (RYGB) were compared with nearly 13,000 patients (average age 55) who had adjustable gastric banding. Over an average follow-up of 3½ years, there were 658 nonvertebral fractures, such as to the wrist, pelvis, upper arm and hip among the patients. The study reports patients who had RYGB had increased risk of fracture compared with patients who had adjustable gastric banding. A limitation of the study is that a large proportion of the study group was eligible for Medicare because of a disability; patients younger than 65 with certain disabilities are eligible for Medicare. Fracture risk may be an unintended consequence of RYGB surgery, which is otherwise associated with many health benefits, and this should be discussed with patients.
Authors: Elaine W. Yu, M.D., M.M.Sc., Massachusetts General Hospital, Boston, and coauthors
(doi:10.1001/jamasurg.2019.1157)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Could Locking All Household Guns Reduce Youth Suicides, Unintentional Firearm Deaths?
JAMA Pediatrics
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, MAY 13, 2019
Media advisory: To contact corresponding author Michael C. Monuteaux, Sc.D., email Erin Tornatore at erin.tornatore@childrens.harvard.edu. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamapediatrics/fullarticle/2733158?guestAccessKey=8248b8a2-3f76-4e0a-b003-2f84f2b4a180 &utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=051319
Bottom Line: An increase in the number of firearm owners who live with children who lock up all their household guns could be associated with a reduction in youth firearm deaths by suicide and unintentional injury. This modeling study of a hypothetical intervention estimates that if 20% of households storing at least one gun unlocked changed in one year to locking all firearms then between 72 and 135 youth firearm fatalities and between 235 and 323 youth firearm shootings (nonfatal injuries and deaths combined) may be prevented. A gun in a home can increase risk of suicide and unintentional deaths, and safe gun storage practices have been associated with reduced risk of intentional self-inflicted and unintentional firearm injuries. Authors of this study acknowledge their analysis relied on a single study from more than 15 years ago because they says it’s the only published study that quantifies the relationship between gun storage practices among youth and the risk of firearm injuries. Nonetheless, researchers say their results underscore the need to develop approaches that will motivate adults to safely store guns.
Authors: Michael C. Monuteaux, Sc.D., of Boston Children’s Hospital, and coauthors
(doi:10.1001/jamapediatrics.2019.1078)
Editor’s Note: The article contains funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Cost-Effectiveness Analysis of 12 Cervical Cancer Screenings
JAMA Internal Medicine
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, MAY 13, 2019
Media advisory: To contact corresponding author George F. Sawaya, M.D., email Elizabeth Fernandez at elizabeth.fernandez@ucsf.edu. The full study, commentary and author interview are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2732691?guestAccessKey=269f58b8-5962-4c16-abe1-fabbd1f76d77&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=051319
Bottom Line: This cost-effectiveness analysis incorporates women’s preferences and estimates quality of life and economic outcomes for 12 cervical cancer screening strategies.
Authors: George F. Sawaya, M.D., of the University of California, San Francisco, and coauthors
(doi:10.1001/jamainternmed.2019.0299)
Editor’s Note: The articles conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Philadelphia Beverage Tax Associated With Higher Prices, Reduced Sales
JAMA
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, MAY 14, 2019
Media advisory: To contact corresponding author Christina A. Roberto, Ph.D., email Katie Delach at Katie.Delach@pennmedicine.upenn.edu. The full study and editorial are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jama/fullarticle/2733208?guestAccessKey=86610f39-a0eb-46d4-a30a-3ddef0036408&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=051419
Bottom Line: A few U.S. cities have instituted beverage taxes on sweetened drinks to generate revenue and to reduce consumption of these drinks because of their association with obesity and poor health. This study looked at changes in beverage prices and sales before and after Philadelphia implemented such a tax (1.5 cents per ounce) in 2017 compared with Baltimore, which had no such tax. In an analysis that included nearly 300 stores, study authors report Philadelphia’s tax was associated with increased beverage prices and large sales declines, which were partially offset by increased purchases in neighboring areas. This study relied on data for beverages sold only at chain retailers.
Authors: Christina A. Roberto, Ph.D., University of Pennsylvania Perelman School of Medicine, Philadelphia, and coauthors
(doi:10.1001/jama.2019.4249)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
What is Association of Age With Risk of Death for ICU Patients?
JAMA Network Open
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, MAY 10, 2019
Media advisory: To contact corresponding author Matthieu Legrand, M.D., Ph.D., email Matthieu.Legrand@aphp.fr. The full study and editorial are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2019.3215?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=051019
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: This study of nearly 134,000 patients admitted to intensive care units in France examined the association of age with risk of death in the hospital and then three months and three years after discharge.
Authors: Matthieu Legrand, M.D., Ph.D., L’Assistance Publique-Hopitaux de Paris, France, and coauthors
(doi:10.1001/jamanetworkopen.2019.3215)
Editor’s Note: The article includes conflict of interest disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Time of Day Associated With Physicians Ordering Cancer Screenings, Patients Completing Them
JAMA Network Open
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, MAY 10, 2019
Media advisory: To contact corresponding author Mitesh S. Patel, M.D., M.B.A., M.S., email Frank Otto at Frank.Otto@pennmedicine.upenn.edu. The full study and commentary are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2019.3403?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=051019
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: The time of day of a primary care appointment was associated with the likelihood of a physician ordering cancer screenings and of patients completing those screenings in this study of 33 practices with patients eligible for breast or colorectal cancer screening. The likelihood of physicians ordering cancer screenings decreased as the clinic day progressed and so did the likelihood of patients completing those screenings within one year of the office visit. This observational study cannot explain the cause behind these associations but clinician and patient factors may explain it, such as shorter interactions with patients if physicians fall behind and cancer screenings not being discussed. Screening test order rates were highest at 8 a.m. and lowest at 5 p.m. The results of this study may not be generalizable because it was conducted at a single health system and variation in screening orders and patient completion may be related to factors unaccounted for in this study. Future interventions that aim to increase cancer screenings should consider how the timing of primary care visits might influence physician and patient behavior.
Authors: Mitesh S. Patel, M.D., M.B.A., M.S., University of Pennsylvania, Philadelphia, and coauthors
(doi:10.1001/jamanetworkopen.2019.3403)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Disparities Associated With Buprenorphine Prescriptions for Opioid Use Disorder
JAMA Psychiatry
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, MAY 8, 2019
Media advisory: To contact corresponding author Pooja A. Lagisetty, M.D., M.Sc., email Kara Gavin at kegavin@med.umich.edu. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamapsychiatry/fullarticle/2732871?guestAccessKey=85d2749b-f483-45ae-ad34-513cfa5584a2&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=05082019
Bottom Line: This study used national survey data to estimate buprenorphine prescription rates to treat opioid use disorder by race/ethnicity and by payment type for office visits, which is how most patients with buprenorphine prescriptions get care. Researchers report buprenorphine office visits increased from 0.04% to 0.36% of ambulatory visits from 2004-2015 and that represented about 13.4 million visits from 2012-2015. Buprenorphine prescriptions were received at more visits from 2012-2015 by white patients than patients of other races/ethnicities. Office visits were most commonly paid for by private insurance or were self-pay. Increasing rates of opioid overdoses mean it is important that policy and research efforts address racial/ethnic and payment differences in access to treatment for opioid use disorder.
Authors: Pooja A. Lagisetty, M.D., M.Sc., University of Michigan, School of Medicine, Ann Arbor, and coauthors
(doi:10.1001/jamapsychiatry.2019.0876)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
How Common is Alcoholic Fatty Liver Disease?
JAMA
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, MAY 7, 2019
Media advisory: To contact corresponding author Robert J. Wong, M.D., M.S., email Terry Lightfoot at tlightfoot@alamedahealthsystem.org. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jama/fullarticle/2732559?guestAccessKey=644408df-7a63-40c2-8e61-3d0988921a38&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=050719
Bottom Line: This study used national survey data from 2001-2016 to examine how common alcoholic fatty liver disease is in the United States.
Authors: Robert J. Wong, M.D., M.S., Highland Hospital, Oakland, California, and coauthors
(doi:10.1001/jama.2019.2276)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Articles Focus on Psoriasis, Risk of Mental Health Disorders
JAMA Dermatology
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, MAY 8, 2019
Media advisory: The full studies and editorial are linked to this news release.
Bottom Line: Two related articles and an editorial focus on the chronic inflammatory skin disorder psoriasis and the risk of mental health disorders.
Embed these links to provide your readers free access to the full-text articles: These links will be live at the embargo time.
Psoriasis and Risk of Mental Disorders in Denmark (Leisner):
Association of Psoriasis With Mental Health Disorders in South Korea (Lee):
Exploring Mental Disorders in Patients With Skin Diseases (Armstrong):
Editor’s Note: Please see the articles for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Association Between Weight Before Pregnancy, Weight Gain During Pregnancy and Adverse Outcomes for Mother, Infant
JAMA
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, MAY 7, 2019
Media advisory: To contact corresponding author Romy Gaillard, M.D., Ph.D., email r.gaillard@erasmusmc.nl. The full study and editor’s note are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jama/fullarticle/2732571?guestAccessKey=a4d9e804-3715-4087-8190-195e8d47c205&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=050719
Bottom Line: An analysis that combined the results of 25 studies including nearly 197,000 women suggests prepregnancy body mass index (BMI) of the mother was more strongly associated with risk of adverse maternal and infant outcomes than the amount of gestational weight gain.
Authors: Romy Gaillard, M.D., Ph.D., Erasmus MC, University Medical Center, Rotterdam, the Netherlands, and coauthors
(doi:10.1001/jama.2019.3820)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
How Common is E-Cigarette Use Among Adults in Households With Kids?
JAMA Pediatrics
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, MAY 6, 2019
Media advisory: To contact corresponding author Jenny L. Carwile, Sc.D., M.P.H., email media@mmc.org. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamapediatrics/fullarticle/2732625?guestAccessKey=2980756e-2eb1-4b37-8ecc-f7628f55876a&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=050619
Bottom Line: Nearly 5% of adults living in households with children use e-cigarettes based on analyses of national survey data from 2016-2017.
Authors: Jenny L. Carwile, Sc.D., M.P.H., of Maine Medical Center, Portland, Maine, and coauthors
(doi:10.1001/jamapediatrics.2019.1139)
Editor’s Note: The article contains conflict of interest disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Aspirin Before At-Home Colorectal Cancer Screening Test Didn’t Significantly Improve Ability to Detect Cancer Precursors
JAMA
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, MAY 7, 2019
Media advisory: To contact corresponding author Hermann Brenner, M.D., M.P.H., email h.brenner@dkfz-heidelberg.de. The full study is linked to this news release and a visual abstract is below.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jama/fullarticle/2732573?guestAccessKey=4603433e-571d-4e10-9155-6e0c558fbecc&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=050719
Bottom Line: Some observational studies have suggested that taking aspirin before undergoing colorectal cancer screening with a fecal immunochemical test for blood in stool might improve the ability of the test to detect cancer precursors. This randomized clinical trial included about 1,200 adults (between the ages of 40 and 80) who took a single dose of aspirin (300 mg) two days before collecting a stool sample for a fecal immunochemical test and about 1,200 adults who took a placebo. Researchers report a higher detection rate in the aspirin group but the difference between the two groups wasn’t statistically significant. A limitation of the study is that the results were based on relatively few individuals who had cancer precursors.
Authors: Hermann Brenner, M.D., M.P.H., German Cancer Research Center, Heidelberg, Germany, and coauthors

(doi:10.1001/jama.2019.4755)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Clinical Trial Looks at Absorption Levels of Sunscreen Active Ingredients into Bloodstream
JAMA
EMBARGOED FOR RELEASE: 11 A.M. (ET), MONDAY, MAY 6, 2019
Media advisory: To contact corresponding author David G. Strauss, M.D., Ph.D., email Sandy Walsh at Sandy.Walsh@fda.hhs.gov. The full study and editorial are linked to this news release and a visual abstract is below.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jama/fullarticle/2733085?guestAccessKey=e1ad4492-fe70-4f53-970d-d63bfa1cdccd&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=05062019
Bottom Line: The U.S. Food and Drug Administration recommends that active ingredients in sunscreen absorbed into the bloodstream above a certain level undergo toxicology testing. Researchers from the FDA conducted this small randomized clinical trial of 24 healthy volunteers to determine bloodstream concentrations of four active ingredients (avobenzone, oxybenzone, octocrylene and ecamsule) in four sunscreens applied four times per day for four days with blood samples collected from study participants over seven days. Researchers report that all four active ingredients were found in blood samples at levels exceeding the threshold recommended for toxicology testing. The effect of these concentrations is unknown and further studies are needed to determine the clinical significance of these findings. Some limitations of this clinical trial include that it was conducted under indoor conditions without exposure to heat, sunlight or humidity, which may affect the rate of sunscreen absorption, and the study wasn’t designed to look at differences in absorption by the type of sunscreen formulation, skin type or age of the user. Researchers emphasize that their results don’t suggest people refrain from using sunscreen, which prevents skin damage.
Authors: David G. Strauss, M.D., Ph.D., U.S. Food and Drug Administration, Silver Spring, Maryland, and coauthors

(doi:10.1001/jama.2019.5586)
Editor’s Note: The article includes funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Severe Tinnitus Associated with Suicide Attempts in Women
JAMA Otolaryngology-Head & Neck Surgery
EMBARGOED FOR RELEASE: 11 A.M. (ET), THURSDAY, MAY 2, 2019
Media advisory: To contact corresponding author Christopher R. Cederroth, Ph.D., email christopher.cederroth@ki.se. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamaotolaryngology/fullarticle/2732497?guestAccessKey=29ff1922-fff3-4f3b-999a-ec8c1aeca212&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=050219
Bottom Line: Previously, severe ringing in the ears (tinnitus) has been associated with depression and anxiety, and a 2016 study reported an association with increased risk of suicide attempts. This study used responses to a questionnaire from about 72,000 adults in Sweden to examine whether an association with increased risk of suicide attempts might be different between men and women. Of 874 women who reported severe tinnitus, 82 (9.4%) reported attempting suicide; of 1,121 men who reported severe tinnitus, 62 (5.5%) reported a suicide attempt. After analyses, an association between severe tinnitus and suicide attempt remained statistically significant only in women. Individuals who had a formal diagnosis of tinnitus weren’t at increased risk for a suicide attempt, suggesting medical attention may help to alleviate quality of life impairments. Cognitive behavioral therapy is an established approach. A limitation of the study is its reliance on self-reported tinnitus.
Authors: Christopher R. Cederroth, Ph.D., Karolinska lnstitutet, Stockholm, Sweden, and coauthors
(doi:10.1001/jamaoto.2019.0566)
Editor’s Note: The article contains conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Study Examines Private Insurance Claims for Naloxone Prescriptions
JAMA Network Open
EMBARGOED FOR RELEASE: 11 A.M. (ET), FRIDAY, MAY 3, 2019
Media advisory: To contact corresponding study author Mai T. Pho, M.D., M.P.H., email Ashley Heher at Ashley.Heher@uchospitals.edu. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamanetworkopen/fullarticle/10.1001/jamanetworkopen.2019.3209?utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_term=050319
About JAMA Network Open: JAMA Network Open is the new online-only open access general medical journal from the JAMA Network. Every Friday, the journal publishes peer-reviewed clinical research and commentary in more than 40 medical and health subject areas. Every article is free online from the day of publication.
Bottom Line: A study based on a national database of private insurance claims suggests few patients at high risk of opioid overdose receive prescriptions for naloxone, which can reverse an overdose, during encounters with the health care system from hospitalizations and emergency department visits to physician visits. Among 138,108 high-risk patients, only 2,135 (1.5%) were prescribed naloxone. A greater likelihood of receiving naloxone was associated with prior diagnoses of opioid misuse or dependence and overdose compared to those diagnoses without overdose. A prior diagnosis of opioid overdose alone was associated with decreased likelihood of receiving naloxone compared with a prior diagnosis of opioid misuse or dependence without overdose. Study authors acknowledge their results underestimate the distribution and use of naloxone because many patients get it through programs where insurance isn’t billed or patients may pay out of pocket. The study findings also may not be generalized to patients covered by Medicaid, some Medicare plans or those who are uninsured. Still, understanding barriers, such as a lack of awareness of local laws, to physician prescribing of naloxone to high-risk patients is important so that health care encounters aren’t missed opportunities to provide the medication.
Authors: Mai T. Pho, M.D., M.P.H., of the University of Chicago Medicine, and coauthors
(doi: 10.1001/jamanetworkopen.2019.3209)
Editor’s Note: The article contains funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Study Looks at Association of High Cholesterol Levels, Statin Use with Glaucoma Risk
JAMA Ophthalmology
EMBARGOED FOR RELEASE: 11 A.M. (ET), THURSDAY, MAY 2, 2019
Media advisory: To contact corresponding author Jae H. Kang, Sc.D., email Mark Murphy at mmurphy90@bwh.harvard.edu. The full study and commentary are linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamaophthalmology/fullarticle/2732293?guestAccessKey=082bacf5-d77a-4215-a06c-78d2f1d8bbb9&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=050219
Bottom Line: A study of adults 40 and older suggests high cholesterol levels are associated with increased risk for the most common form of glaucoma, while longer use of a cholesterol-lowering statin, compared with never using, was associated with lower risk. Data for this observational study came from more than 136,000 adults who participated in three national study groups and provided information on their statin use and cholesterol levels over 15 years. There were 886 new cases of primary open-angle glaucoma (POAG) identified among the adults. The association between longer statin use for five or more years and lower risk of POAG was stronger among those 65 and older. The study is limited by self-reported statin use and cholesterol levels, and the results also may not generalize to other groups because study participants were mostly white health care professionals. These results need to be confirmed in other studies but they are of interest given the widespread use of statins in older persons at risk for this type of glaucoma.
Authors: Jae H. Kang, Sc.D., Brigham and Women’s Hospital, Harvard Medical School, Boston, and coauthors
(doi:10.1001/jamaophthalmol.2019.0900)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
Could Mouth Rinse to Detect HPV DNA Be Associated With Predicting Risk of Head/Neck Cancer Recurrence, Death?
JAMA Oncology
EMBARGOED FOR RELEASE: 11 A.M. (ET), THURSDAY, MAY 2, 2019
Media advisory: To contact corresponding author Maura L. Gillison, M.D., Ph.D., email Scott Merville at SMerville@mdanderson.org. The full study is linked to this news release.
Embed this link to provide your readers free access to the full-text article: This link will be live at the embargo time https://jamanetwork.com/journals/jamaoncology/fullarticle/2732508?guestAccessKey=c77c5154-8844-4d11-9f85-c10579e8a319&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=050219
Bottom Line: Researchers examined if a mouth rinse to detect human papillomavirus (HPV) DNA might be associated with helping to predict risk of recurrence of head and neck squamous cell cancer and death. This study included 396 adults with head and neck squamous cell cancer of the mouth or throat, of which 202 patients had HPV-positive cancers. The adults were tested for the presence of HPV DNA with an oral rinse at various times. After completing cancer treatment, the repeated detection of HPV DNA identical to their tumor type in oral rinses was associated with a higher risk of cancer recurrence and death. The typical follow-up time of about two years in this study may have underestimated the associations between the persistence of HPV and cancer recurrence. More research is needed but these findings suggest HPV DNA may be a promising biomarker to understand cancer treatment response and future risk of progression.
Authors: Maura L. Gillison, M.D., Ph.D., University of Texas MD Anderson Cancer Center, Houston, and coauthors
(doi:10.1001/jamaoncol.2019.0439)
Editor’s Note: The article includes conflict of interest and funding/support disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.
