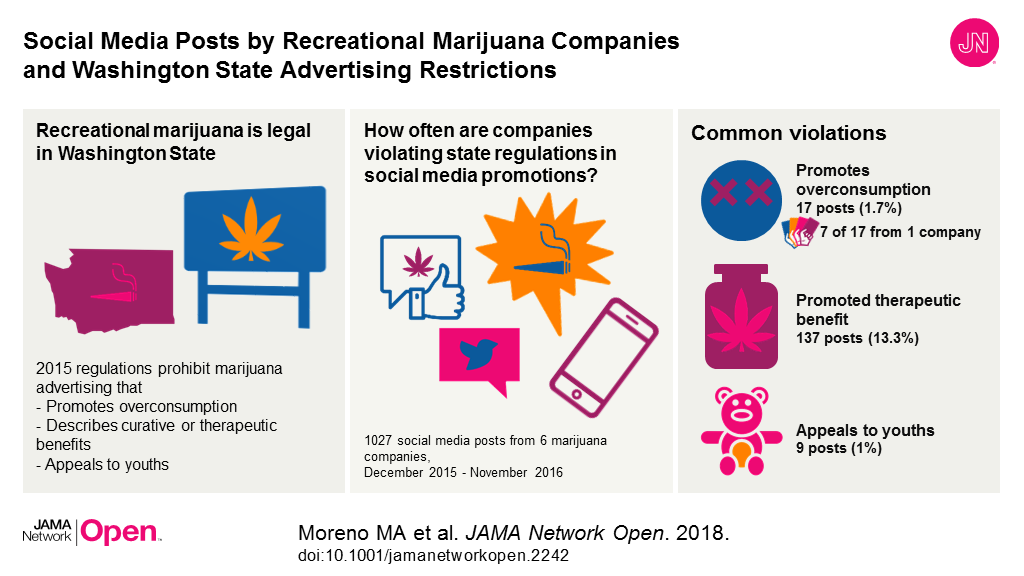
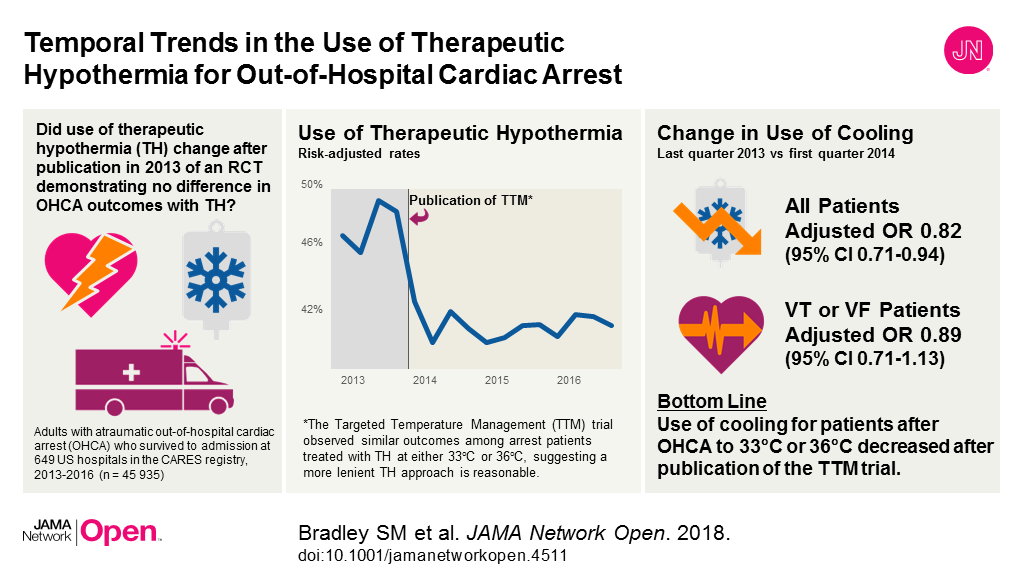
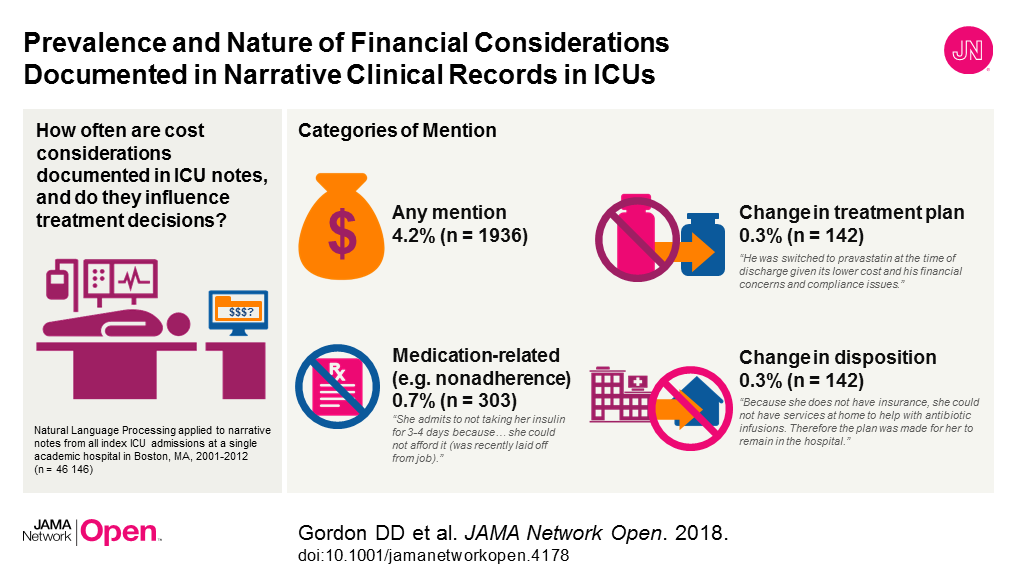
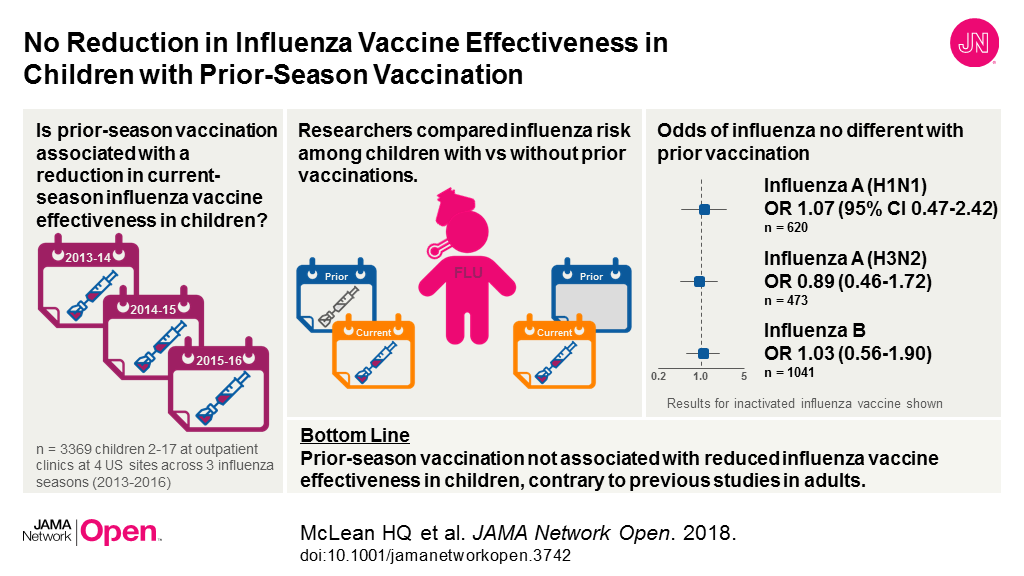
JAMA Cardiology
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, NOVEMBER 28, 2018
Media advisory: To contact corresponding author Deepak L. Bhatt, M.D., M.P.H., email Johanna Younghans at jyounghans@bwh.harvard.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jamacardiology/fullarticle/10.1001/jamacardio.2018.3951
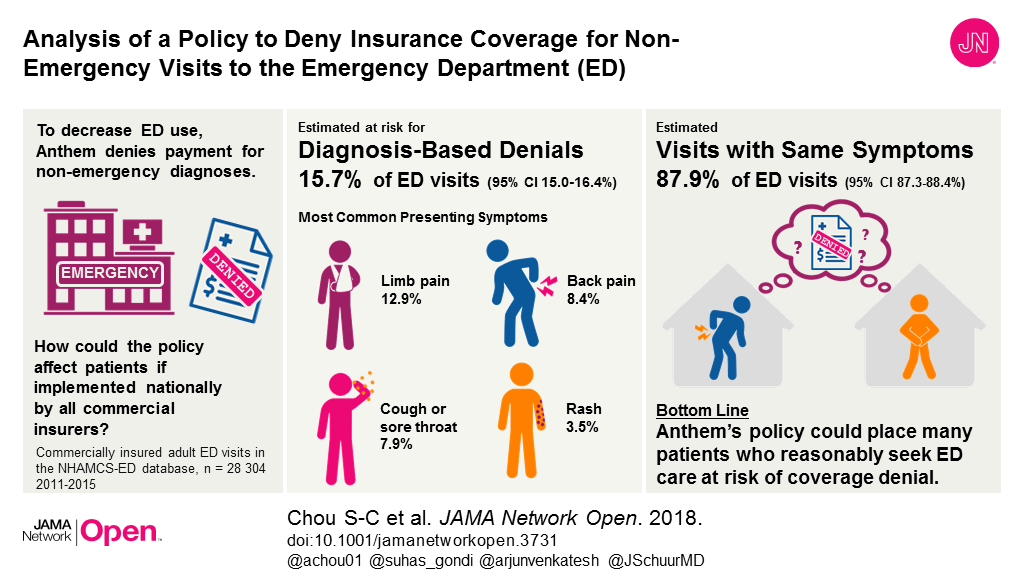
Bottom Line: Whether hospital rankings by U.S. News & World Report magazine reflect quality of care has been debated. This study examined if the magazine’s 50 top-ranked hospitals for cardiovascular care performed better than 3,500 non-ranked hospitals on death rates and hospital readmissions for three cardiovascular conditions, as well as patient satisfaction. Researchers report top-ranked hospitals did have lower 30-day mortality rates for heart attack, heart failure and coronary artery bypass grafting (CABG) and higher patient satisfaction ratings compared with non-ranked hospitals. However, 30-day readmission rates were either similar (for heart attack and CABG) or higher (for heart failure) at the top-ranked hospitals compared with non-ranked hospitals. The discrepancy between readmissions and other measures raises concern that readmissions may not be an adequate metric of hospital care quality. A limitation of the study was that the rankings only include data from Medicare patients.
Authors: Deepak L. Bhatt, M.D., M.P.H., Brigham and Women’s Hospital, Harvard Medical School, Boston, and coauthors
To Learn More: The full study is available on the For The Media website.
(doi:10.1001/jamacardio.2018.3951)
Editor’s Note: The article includes conflict of interest disclosures. Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.