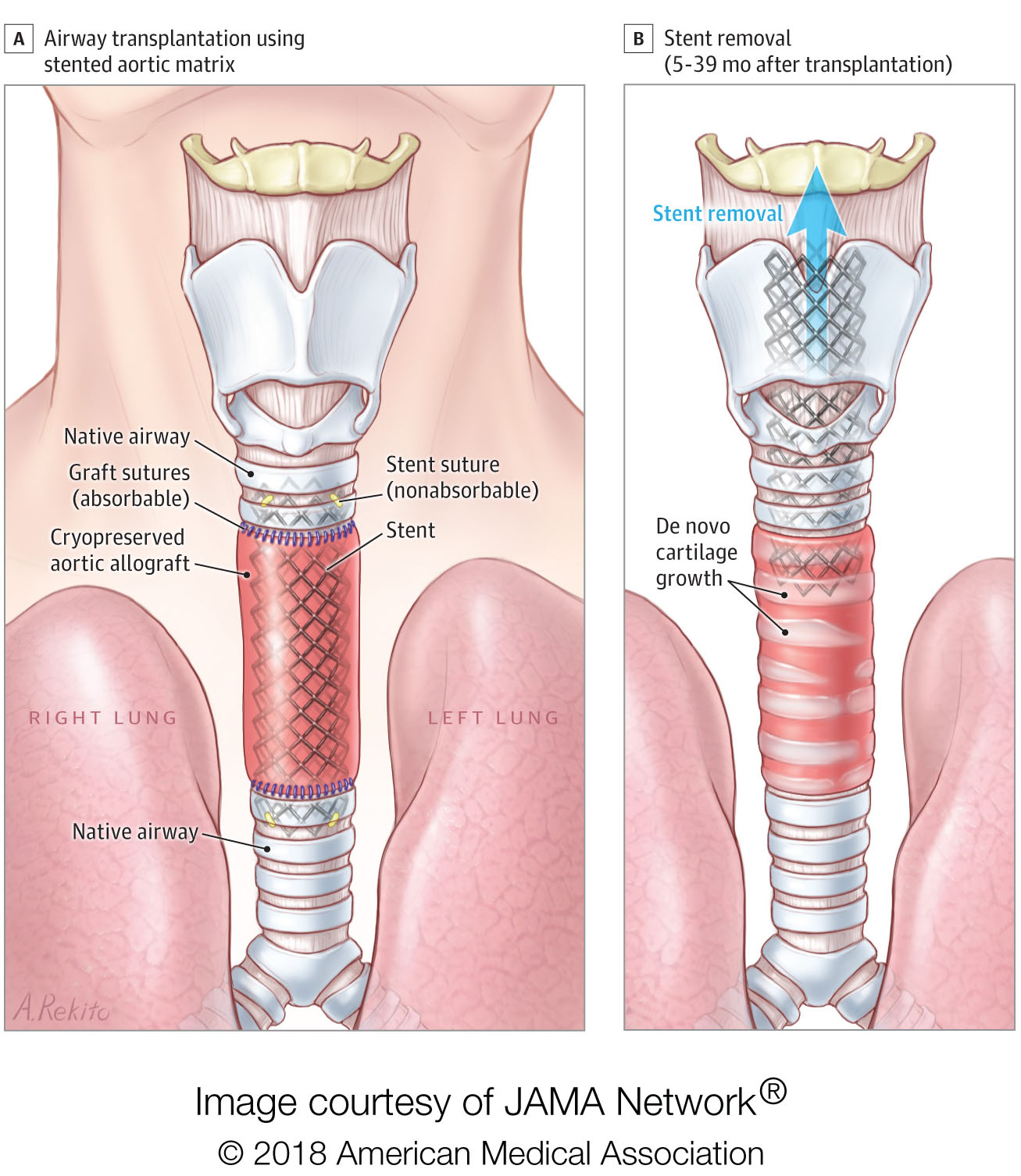
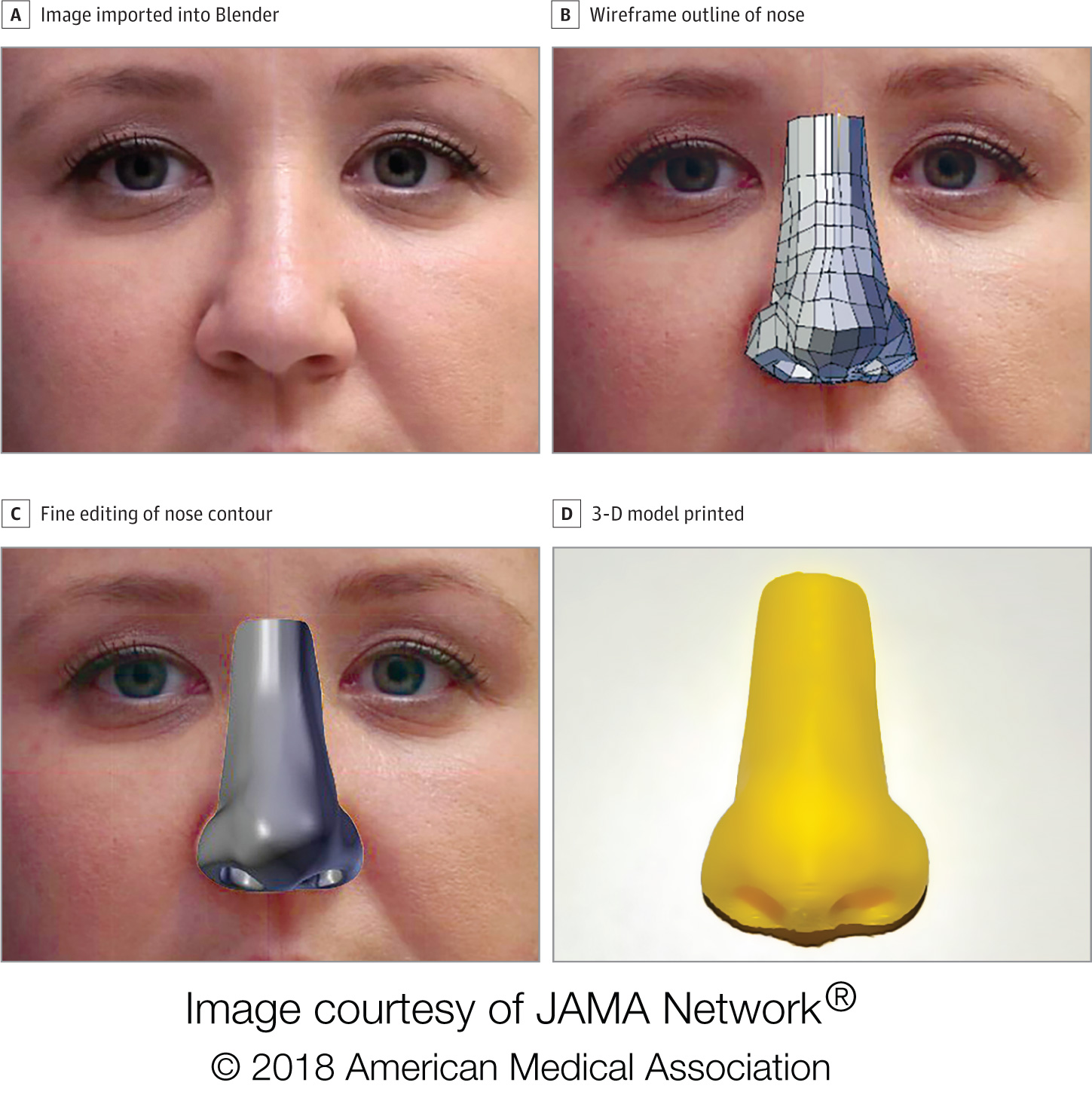
JAMA Surgery
EMBARGOED FOR RELEASE: 11 A.M. (ET), WEDNESDAY, JUNE 20, 2018
Media advisory: To contact corresponding author Andrea L. Pusic, M.D., M.H.S., email Elaine St. Peter at estpeter@bwh.harvard.edu. To contact corresponding author Edwin G. Wilkins, M.D., M.S., email Nicole Fawcett at nfawcett@umich.edu. The full studies are available on the For The Media website.
Bottom Line: Two studies, a commentary and podcast focus on patient outcomes after breast reconstruction surgery following mastectomy. One studied compared satisfaction and quality of life between patients who had breast reconstruction using implants or their own tissue, and a second study compared two-year complication rates across common breast reconstruction techniques.
What: Women who had breast reconstruction after mastectomy using their own tissue (autologous reconstruction) were more satisfied two years later with their breasts and had greater sexual and psychosocial well-being than patients who had reconstruction with implants.
Authors: Andrea L. Pusic, M.D., M.H.S., Brigham Health, Boston, and coauthors
Want to embed a link to this study in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jamasurgery/fullarticle/10.1001/jamasurg.2018.1677
(doi:10.1001/jamasurg.2018.1677)
What: While nearly one-third of women undergoing breast reconstruction following mastectomy experienced complications, failure of the reconstruction occurred in only 5.4 percent. Patients having reconstruction with their own tissue (autologous) were more likely to develop complications but also had lower chances of failure compared with patients choosing implant techniques.
Authors: Edwin G. Wilkins, M.D., M.S., University of Michigan, Ann Arbor, and coauthors
Want to embed a link to this study in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jamasurgery/fullarticle/10.1001/jamasurg.2018.1687
(doi:10.1001/jamasurg.2018.1687)
Related Material:
— A podcast with Drs. Pusic and Wilkins is available for listening and downloading on this page.
— The commentary, “Autologous vs Prosthetic Breast Reconstruction,” by Kenneth L. Fan, M.D., and David H. Song, M.D., M.B.A., MedStar Georgetown University Hospital, Washington, D.C., is also available on the For The Media website.
To Learn More: The full studies are available on the For The Media website.
Editor’s Note: Please see the articles for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.