JAMA
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, APRIL 17, 2018
Media advisory: To contact the U.S. Preventive Services Task Force, email the Media Coordinator at Newsroom@USPSTF.net or call 202-572-2044. The full report is available on the For The Media website.
Want to embed a link to this report in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jama/fullarticle/10.1001/jama.2018.3097
Bottom Line: For adults 65 years or older who are at increased risk of falling, the U.S. Preventive Services Task Force (USPSTF) recommends exercise, such as supervised individual and group classes and physical therapy, to prevent falls, and that clinicians selectively check older adults’ risks for falls and then offer tailored interventions that address those specific risks. The USPSTF recommends against vitamin D supplementation.
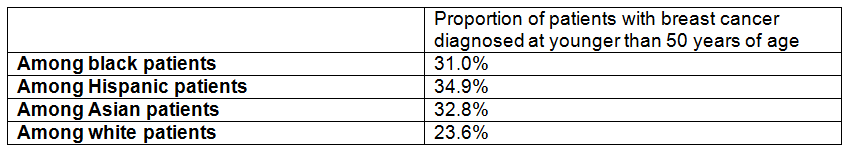
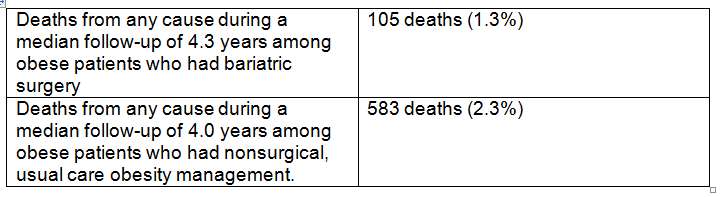
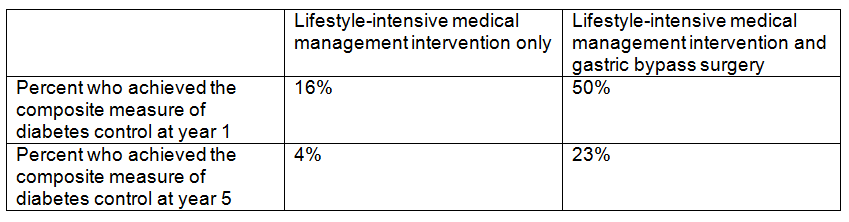
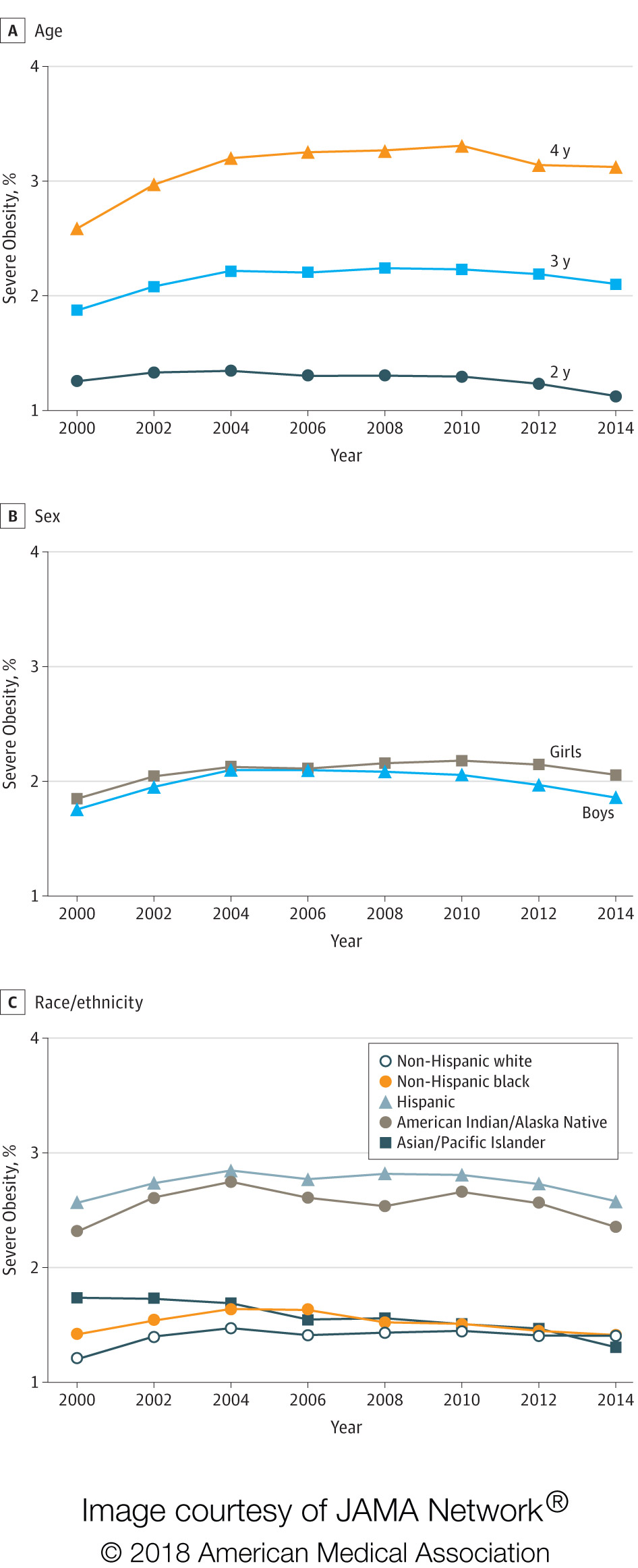
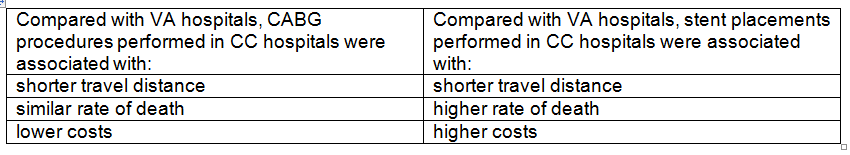
Background: The USPSTF routinely makes recommendations about the effectiveness of preventive care services. This latest recommendation statement is an update of the 2012 recommendation on the prevention of falls in older adults living in the community. Falls are the leading cause of injury-related illness and death among older adults in the United States. In 2014, nearly 30 percent of community-dwelling adults 65 or older reported falling, resulting in 29 million falls and an estimated 33,000 deaths in 2015.
The USPSTF Concludes:

Related material
The following related elements from The JAMA Network are also available on the For The Media website:
— A podcast interview with Alex H. Krist, M.D., M.P.H., a member of the USPSTF and co-author of the recommendation statements regarding calcium and vitamin D to prevent fractures and interventions to prevent falls in community-dwelling adults.
— Interventions to Prevent Falls in Older Adults – Updated Evidence Report and Systematic Review for the US Preventive Services Task Force
— JAMA editorial: Preventing Fractures and Falls
— JAMA Internal Medicine editorial: New Prevention Guidelines for Falls and Fractures
For more details and to read the full report, please visit the For The Media website.
(doi:10.1001/jama.2018.3097)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
Note: More information about the U.S. Preventive Services Task Force, its process, and its recommendations can be found on the newsroom page of its website.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.