JAMA
EMBARGOED FOR RELEASE: 11 A.M. (ET), TUESDAY, DECEMBER 5, 2017
Media advisory: To contact corresponding author Rahi Abouk, Ph.D., email Theresa Ross at RossT@wpunj.edu. The full study is available on the For The Media website.
Want to embed a link to this study in your story? Link will be live at the embargo time https://jamanetwork.com/journals/jama/fullarticle/10.1001/jama.2017.17627
Bottom Line: Statewide implementation of mandatory policies to screen newborns for the most serious congenital heart defects was associated with an estimated decrease in infant cardiac deaths.
Why The Research Is Interesting: Congenital heart disease accounted for 6 percent of U.S. infant deaths from 1999 to 2006. In 2011, critical congenital heart disease was added to the U.S. Recommended Uniform Screening Panel for newborns but it is not known if the policy requiring screen for the most serious defects is associated with lower infant death rates.
Who and When: Infants born between 2007 and 2013.
What (Study Measures): Before-after comparison of early infant deaths (between 24 hours and 6 months of age) from critical congenital heart disease or other congenital cardiac causes in states with mandatory vs nonmandatory screening policies. As of June 2013, eight states had implemented mandatory screening policies, five states had voluntary screening policies, and nine states had adopted but not yet implemented mandates.
How (Study Design): This is an observational study. Researchers are not intervening for purposes of the study so they cannot control natural differences that could explain the study findings.
Authors: Rahi Abouk, Ph.D., William Paterson University, Wayne, New Jersey, and coauthors.
Results: There was a decrease in estimated infant cardiac death rates for states that implemented mandatory screening policies compared to states that did not.
Featured Image:
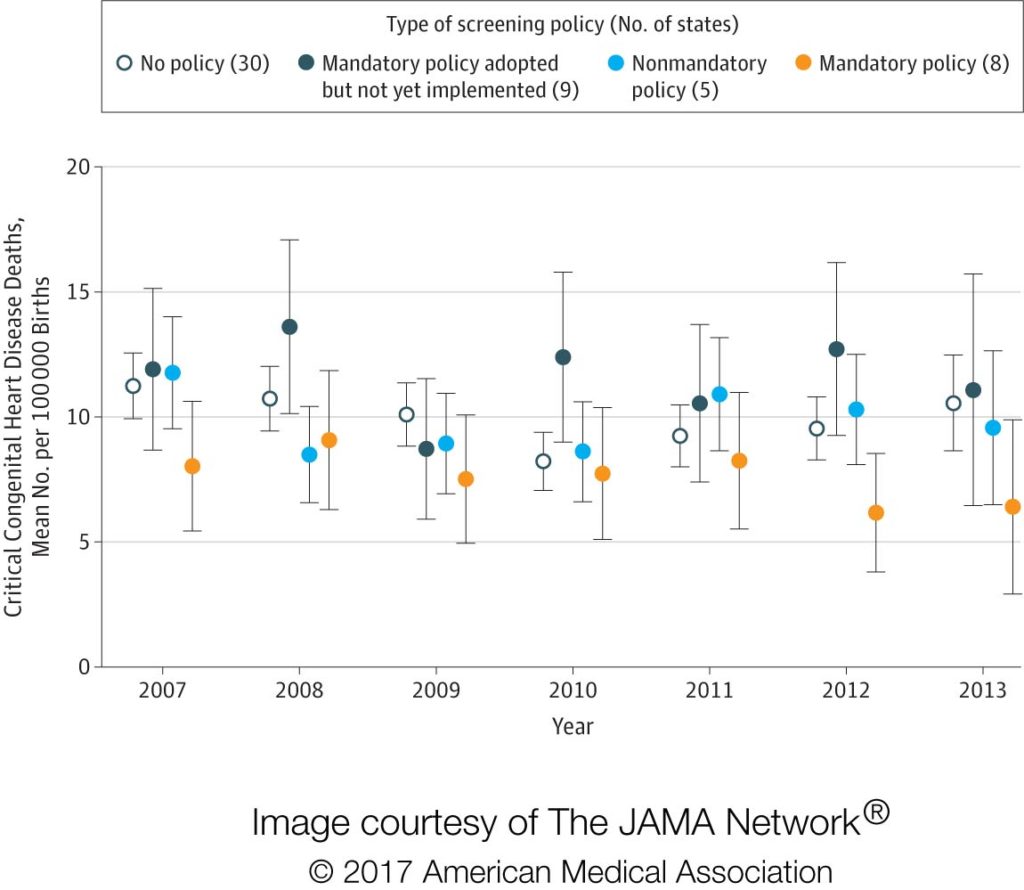
What The Image Shows: States with mandatory screening policies (yellow dots) had lower critical congenital heart disease deaths than states without, and the between-state differences increased after implementation of the screening requirement in 2011. (Click on the image for a full-size version. Right click to “save image as” to download.)
Study Limitations: Estimates of death rates were imprecise because of the small number of deaths and the small number of states with fully implemented screening mandates.
Study Conclusions: The findings support the use of statewide policies requiring newborn critical congenital heart disease screening as one means to reduce U.S. infant mortality.
Related material:
The following related elements also are available on the For The Media website:
The editorial, “The Success of State Newborn Screening Policies for Critical Congenital Heart Disease,” by Alex R. Kemper, M.D., M.P.H., M.S., of Nationwide Children’s Hospital, Columbus, Ohio, and coauthors.
For more details and to read the full study, please visit the For The Media website.
(doi:10.1001/jama.2017.17627)
Editor’s Note: Please see the article for additional information, including other authors, author contributions and affiliations, financial disclosures, funding and support, etc.
# # #
For more information, contact JAMA Network Media Relations at 312-464-JAMA (5262) or email mediarelations@jamanetwork.org.